Flipping the Script: Could Myelin Degeneration Drive Amyloidosis?
Quick Links
As the brain ages, the insulating myelin sheath around axons begins to degrade. Could this white-matter degeneration trigger Alzheimer’s disease? In a preprint on bioRxiv, researchers led by Constanze Depp and Klaus-Armin Nave at the Max Planck Institute of Experimental Medicine in Göttingen, Germany, suggest as much. In mouse models of amyloidosis, myelin damage accelerated plaque formation, while a lack of myelin delayed it. The authors identified two mechanisms linking myelin to plaques in mouse brain. One, damaged myelin drove production of Aβ, directly leading to deposits; two, microglia appeared to preferentially mop up degenerating myelin, ignoring plaques and allowing them to grow. The data hint that myelin damage could tip a brain toward AD, and may even explain why Alzheimer’s risk increases with age, Depp told Alzforum. If the finding holds in people, it will imply that protecting myelin could help prevent AD, she added.
- Myelin defects speed up amyloid deposition in mice, while lack of myelin delays it.
- Degenerating myelin drives Aβ production.
- Damaged myelin distracts microglia from plaques.
Others said the data advance the field. “This fascinating paper by Depp et al. greatly advances our understanding of how oligodendrocytes contribute to AD,” Mikael Simons at Technical University Munich, Germany, wrote to Alzforum (full comment below). Barbara Bendlin at the University of Wisconsin, Madison, pointed to previous hints connecting myelin to Alzheimer’s pathology. “The results greatly strengthen the myelin/AD link, and will be highly encouraging to researchers focused on understanding white-matter abnormalities in AD,” she wrote (comment below).

Plaque Pusher. 5xFAD mice with myelin defects (right) develop 50 percent more cortical plaques (white) than do 5xFAD controls (left). [Courtesy of Depp et al., bioRxiv.]
Researchers have long known that myelin frays during aging, and even more in AD brain (Roher et al., 2002; Stricker et al., 2009; Bowley et al., 2010). Notably, brain regions that start out with the thinnest myelin, such as the frontal cortex, are also the most vulnerable to amyloid pathology (Braak and Braak, 1997). The late George Bartzokis, when he was at the University of California, Los Angeles, proposed a “myelin model” of the brain, in which amyloid deposits might be a by-product of myelin repair (Bartzokis, 2011). Supporting a link, a recent study by Bendlin and colleagues correlated myelin deterioration with Alzheimer’s cerebrospinal fluid biomarkers in people at risk for cognitive decline, implying that white-matter damage could be an early sign of the disorder (Nov 2016 news).
This body of research led the researchers in Germany to ask if myelin deterioration could drive amyloidosis. To explore this, joint first authors Depp and Ting Sun used two myelin mouse models developed in their lab. In one, knockout of the key myelin component proteolipid protein 1 (PLP1) renders the insulating sheath unstable and prone to break down with age (Klugmann et al., 1997). In the other, knockout of the enzyme 2',3'-cyclic nucleotide 3' phosphodiesterase (CNP) prevents oligodendrocytes from metabolically supporting axons. As a consequence, portions of the axon swell up and neurons die (Lappe-Siefke et al., 2003). The authors reasoned that these knockouts might mimic subtle myelin defects that occur with age.
They crossed these mice with 5xFAD mice. At six months of age, 5xFAD mice have widespread cortical plaques. Lack of either PLP1 or CNP worsened this, with 50 percent more plaques in the cortex (see image above). The effect was more pronounced in white matter. In the alveus, a tract overlying the hippocampus, more than twice as many plaques speckled the tissue. This was not a quirk of the 5xFAD model; the authors found a similar boost in plaque formation when they crossed APPNLGF knock-ins with CNP knockouts.
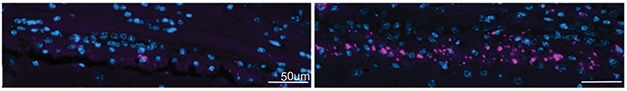
Demyelination Triggers Plaque. In 5xFAD mice injected with a demyelinating agent (right), more plaques (purple) form in the alveus tract than in 5xFAD controls (left). Nuclei are blue. [Courtesy of Depp et al., bioRxiv.]
Other experiments strengthened the evidence. Treating young 5xFAD mice with the demyelinating agent cuprizone boosted plaques in the alveus fourfold (see image above). Likewise, injecting young 5xFAD mice with myelin oligodendrocyte glycoprotein (MOG) to induce demyelination in the spinal cord triggered robust plaque formation around the lesion. Conversely, crossing 5xFAD mice with transgenic mice that lack forebrain myelin delayed plaque formation there by three months.
Myelin M.O.
How does malfunctioning myelin promote amyloidosis? Possibly by boosting Aβ production. In 5xFAD/CNP-/- crosses, swollen myelin around plaques contained about 50 percent more amyloidogenic APP cleavage products and Aβ than did myelin in 5xFAD controls, suggesting more Aβ was made near damaged myelin. Other studies have linked disrupted axonal transport to amyloid production (Oct 2016 conference news; Mar 2017 news; Gowrishankar et al., 2017).
However, microglia changes seemed to play a role as well. In 5xFAD and APPNLGF mice sans CNP, microglia failed to surround plaques. This was reminiscent of TREM2 knockouts, in which microglia lose the ability to corral amyloid (Aug 2017 news; Jan 2019 news). To the authors’ surprise, however, RNA sequencing showed that TREM2 induction was unchanged by loss of CNP. Moreover, microglia in 5xFAD/CNP-/- turned up expression of other disease-associated microglia (DAM) genes even higher than did cells in 5xFAD controls.
Why, then, did they not surround plaques? Perhaps because they were distracted by myelin debris. Depp noted that myelin decays before plaques form in 5xFAD/CNP-/- mice, so microglia may become specialized for myelin cleanup and unable to respond to plaque. In one sign of this, these cells expressed many genes involved in metabolizing lipids, which are abundant in myelin. The microglia appeared similar to the recently described white-matter-associated microglia, Depp said (Mar 2021 news).
Next, the authors want to figure out which mechanism predominates. They will try to parse oligodendrocyte and microglia effects by using myelin mutants that do not activate microglia, and by depleting microglia from 5xFAD/CNP-/- mice. Depp is also interested in the role of tau pathology, and is crossing tauopathy models with the myelin mutants.

Myelin Mop-Up. In AD brain (top), activated microglia (purple) busy themselves cleaning up deteriorating myelin (gold), but ignore amyloid plaques (blue); in age-matched control brain (bottom), myelin is plentiful and microglia scarce. [Courtesy of Depp et al., bioRXiv.]
Does all this happen in human brain? The authors found a large number of activated microglia among deteriorating myelin in postmortem AD brain, but not in age-matched controls (see image at right). In addition, a previous analysis of gene expression around plaques in postmortem AD brain had shown a decrease in myelination genes, again invoking a connection between myelination and plaques (Jul 2020 news).
It is unclear if demyelinating diseases such as multiple sclerosis lead to AD, since until recently people with these conditions did not live long enough to develop diseases of aging. One recent review notes several cases of people with both disorders, but the relative risk of AD in MS patients is unknown (Luczynski et al., 2019). However, cognitive decline is common, affecting more than half of MS patients (National MS Society).
If myelin dysfunction does increase AD risk, would not healthy myelin help stave off the disease? Depp noted that many behaviors that lower AD risk, such as exercise and eating a Mediterranean diet rich in fish oil, are known to benefit myelin (Apr 2006 news; Aug 2016 news; Apr 2017 conference news). On the other hand, environmental factors that damage myelin, such as traumatic brain injury, are associated with a higher risk of AD (Sep 2016 news; May 2018 news).
Could interventions that promote myelination, such as drugs for multiple sclerosis, help keep the brain healthy? Depp is encouraged by recent research showing that administering a myelin-promoting drug to APP/PS1 mice improved their memories and learning abilities (Chen et al., 2021). “Those data were stunning. It’s the other side of the coin from our study, and fits it perfectly,” Depp said.—Madolyn Bowman Rogers
References
News Citations
- Imaging Myelin: Shining a Light on Early Alzheimer’s Pathology?
- Does BACE Drive Neurites into Dystrophy, Shorting Circuits?
- Transport Breakdown Maroons BACE1 in Synapses, Boosts Aβ
- Without TREM2, Microglia Run Out of Gas
- Without TREM2, Plaques Grow Fast in Mice, Have Less ApoE
- WAM! New Microglial Subtype Mops Up Moribund Myelin
- Paper Alert: Those PIGs! Spatial Transcriptomics Add Human Data
- Mediterranean Diet Slims Down Risk of AD
- Less Salmon, More Plaques? Link Between Omega-3s and Aβ Reinvigorates Fish Oil Debate
- NIH Summit Examines What Makes a Healthy Aging Brain
- Axon Damage May Hinder Recovery from Concussion, Spark Neurodegeneration
- Do Mild Traumatic Brain Injuries Double the Risk of Dementia?
Research Models Citations
Paper Citations
- Roher AE, Weiss N, Kokjohn TA, Kuo YM, Kalback W, Anthony J, Watson D, Luehrs DC, Sue L, Walker D, Emmerling M, Goux W, Beach T. Increased A beta peptides and reduced cholesterol and myelin proteins characterize white matter degeneration in Alzheimer's disease. Biochemistry. 2002 Sep 17;41(37):11080-90. PubMed.
- Stricker NH, Schweinsburg BC, Delano-Wood L, Wierenga CE, Bangen KJ, Haaland KY, Frank LR, Salmon DP, Bondi MW. Decreased white matter integrity in late-myelinating fiber pathways in Alzheimer's disease supports retrogenesis. Neuroimage. 2009 Mar 1;45(1):10-6. PubMed.
- Bowley MP, Cabral H, Rosene DL, Peters A. Age changes in myelinated nerve fibers of the cingulate bundle and corpus callosum in the rhesus monkey. J Comp Neurol. 2010 Aug 1;518(15):3046-64. PubMed.
- Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997 Jul-Aug;18(4):351-7. PubMed.
- Bartzokis G. Alzheimer's disease as homeostatic responses to age-related myelin breakdown. Neurobiol Aging. 2011 Aug;32(8):1341-71. PubMed.
- Klugmann M, Schwab MH, Pühlhofer A, Schneider A, Zimmermann F, Griffiths IR, Nave KA. Assembly of CNS myelin in the absence of proteolipid protein. Neuron. 1997 Jan;18(1):59-70. PubMed.
- Lappe-Siefke C, Goebbels S, Gravel M, Nicksch E, Lee J, Braun PE, Griffiths IR, Nave KA. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat Genet. 2003 Mar;33(3):366-74. PubMed.
- Gowrishankar S, Wu Y, Ferguson SM. Impaired JIP3-dependent axonal lysosome transport promotes amyloid plaque pathology. J Cell Biol. 2017 Oct 2;216(10):3291-3305. Epub 2017 Aug 7 PubMed.
- Luczynski P, Laule C, Hsiung GR, Moore GR, Tremlett H. Coexistence of Multiple Sclerosis and Alzheimer's disease: A review. Mult Scler Relat Disord. 2019 Jan;27:232-238. Epub 2018 Oct 27 PubMed.
- Chen JF, Liu K, Hu B, Li RR, Xin W, Chen H, Wang F, Chen L, Li RX, Ren SY, Xiao L, Chan JR, Mei F. Enhancing myelin renewal reverses cognitive dysfunction in a murine model of Alzheimer's disease. Neuron. 2021 Jul 21;109(14):2292-2307.e5. Epub 2021 Jun 7 PubMed.
External Citations
Further Reading
News
- It’s a Wrap: Uncovering Signals Behind Myelination
- Wrap it Up! Neuregulin Directs Axonal Myelination
- Surprise! Astrocytes Mediate Activity-Stimulated Myelination
- Dead Microglia Pave the Way for Myelin Regeneration
- Stem Cells Make New Myelin, Show Potential for AD
- Massive Proteomics Studies Peg Glial Metabolism, Myelination, to AD
- Oligodendrocytes More Than Myelinators, Potential Players in ALS
- Newly Identified Microglia Contain Lipid Droplets, Harm Brain
- Microglia Instigate ‘Chemofog’ by Squelching Myelination
- New Myelin Makes Memories, but Supply Wanes with Age
Primary Papers
- Depp C, Sun T, Sasmita AO, Spieth L, Berghoff SA, Steixner-Kumar AA, Subramanian S, Möbius W, Göbbels S, Saher G, Zampar S, Wirths O, Thalmann M, Saito T, Saido T, Krueger-Burg D, Kawaguchi R, Willem M, Haass C, Geschwind D, Ehrenreich H, Stassart R, Nave KA. Ageing-associated myelin dysfunction drives amyloid deposition in mouse models of Alzheimer’s disease. bioRxiv. August 2, 2021
Annotate
To make an annotation you must Login or Register.

Comments
Technical University Munich
This fascinating paper by Depp et al. greatly advances our understanding of how oligodendrocytes contribute to AD, and shows that there are clearly far more cell types involved in AD than previously suspected.
Myelin undergoes substantial pathological alterations during normal aging. A further understanding of such age-related myelin and its associated cellular responses is essential, as aging is a major risk factor for the most prevalent neurodegenerative diseases such as AD.
In this interesting study, Depp et al. investigated whether myelin degeneration is associated with changes in Aβ deposition by combining mouse models of AD with models of genetically induced hypomyelination or dysmyelination. They observed that in models in which myelin is present but dysfunctional, amyloid deposition is strongly induced. Interestingly, in models in which compact myelin is lacking, less amyloid is deposited. Thus, the authors conclude that defective myelin is an upstream factor of amyloid plaque deposition.
The authors come up with two mechanisms for how myelin defects drive amyloidosis. The first is based on increased APP processing within dysfunctional axons. The second is on the altered microglial responses induced by dys- and demyelination. The authors propose that microglia, once engaged in the clearance of defective myelin, are distracted from amyloid plaques.
This is clearly an important paper as it links myelin dysfunction to amyloid deposition, and provides plausible mechanisms of how this might occur.
George Bartzokis was one of the first who postulated in a series of review papers that myelin breakdown could be connected to AD pathology. He proposed in his opinion papers that myelin-associated factors such as iron could be key in promoting toxicity and depositing amyloid. However, the fact that myelin is enriched in the white matter whereas amyloid plaque deposition occurs predominantly in the gray matter had always been an argument in contradiction to his model.
Yet, it is important to remember that myelin is not exclusive to the white matter. A substantial fraction of the axons within the gray matter is myelinated, and Depp et al. studied in particular the intracortical myelin in their paper. Their findings suggest that age-dependent loss of myelin integrity as it occurs in the gray and white matter under normal aging can be a risk factor for amyloid deposition.
Together with work from Bart de Strooper’ s lab (Chen et al., 2020) which identified myelin/oligodendrocyte gene responses around early amyloid plaques, the study by Depp et al. suggest that myelin dysfunction could be an early event in amyloid plaque deposition.
References:
Chen WT, Lu A, Craessaerts K, Pavie B, Sala Frigerio C, Corthout N, Qian X, Laláková J, Kühnemund M, Voytyuk I, Wolfs L, Mancuso R, Salta E, Balusu S, Snellinx A, Munck S, Jurek A, Fernandez Navarro J, Saido TC, Huitinga I, Lundeberg J, Fiers M, De Strooper B. Spatial Transcriptomics and In Situ Sequencing to Study Alzheimer's Disease. Cell. 2020 Aug 20;182(4):976-991.e19. Epub 2020 Jul 22 PubMed.
University of Wisonsin
There have been several hints over the years that myelin and AD pathology are related. Braak and Braak made an early and key observation that brain regions that myelinate later in development are more vulnerable to development of AD pathology, and several neuroimaging studies have pointed toward white-matter degeneration and abnormalities across the continuum of AD. George Bartzokis was a strong proponent of the idea that myelin degeneration contributes to amyloid pathology and proposed a comprehensive model linking myelin to AD (among other disorders).
Our research group found that, among cognitively unimpaired middle-aged and older adults, cerebrospinal fluid levels of amyloid were related to myelin content measured with MRI. However, studying the link between myelin and AD pathology in humans is tricky. Neuroimaging studies of myelin are particularly challenging, given that myelin degeneration in aging and disease is also accompanied by regeneration (making it difficult to measure injury), and because it's challenging to establish the temporal ordering of events that occur in AD (myelin degeneration in relation to amyloid, tau, and neuronal injury/loss).
In this study, Depp et al. cleverly manipulated myelin in mice to better understand the direct links between myelin and amyloid. Based on a series of experiments with several mouse models ranging from those with myelin defects, to those where myelin was depleted, they found that amyloid deposition could either be enhanced or reduced. Perhaps one of the most intriguing observations to come from this series of experiments is in regard to the role of microglia, with the experiments suggesting that microglia busy themselves with myelin abnormalities to the point that they no longer engage in amyloid clearance. This is a completely new and fascinating piece of the myelin/AD puzzle.
Overall, the results greatly strengthen the myelin/AD link and will be highly encouraging to researchers focused on understanding white-matter abnormalities in AD.
ADvantage
This elegant report further supports the role of myelin breakdown in age-related neurodegeneration. We studied, for over three decades, the age-related changes that occur in the rhesus monkeys that lead to cognitive decline in otherwise healthy primates. The most striking changes seen at the biochemical, molecular biological, and ultrastructural levels were neuroinflammation in the white matter and the degeneration of myelin. Activated microglia and reactive astrocytes were seen only in the white matter of aged monkeys, but not in the gray matter (Sloane et al., 1999; Sloane et al., 2000). Activated microglia contained myelin debris, as seen by EM.
Only in old monkeys did we observe degraded myelin, including degraded CNPase, a major myelin protein (Sloane et al., 2003; Hinman et al., 2004; Hinman et al., 2008). Both calpain and the complement system were involved in myelin deterioration (Duce et al., 2006; Hinman and Abraham, 2007). Thus, just normal aging is enough to trigger myelin breakdown that, in a vicious cycle, is caused by, and further induces, microglia activation and phagocytosis. The hypothesis that microglia are ingesting myelin debris and not Aβ in the AD brain makes perfect sense.
References:
Sloane JA, Hollander W, Moss MB, Rosene DL, Abraham CR. Increased microglial activation and protein nitration in white matter of the aging monkey. Neurobiol Aging. 1999 Jul-Aug;20(4):395-405. PubMed.
Sloane JA, Hollander W, Rosene DL, Moss MB, Kemper T, Abraham CR. Astrocytic hypertrophy and altered GFAP degradation with age in subcortical white matter of the rhesus monkey. Brain Res. 2000 Apr 17;862(1-2):1-10. PubMed.
Sloane JA, Hinman JD, Lubonia M, Hollander W, Abraham CR. Age-dependent myelin degeneration and proteolysis of oligodendrocyte proteins is associated with the activation of calpain-1 in the rhesus monkey. J Neurochem. 2003 Jan;84(1):157-68. PubMed.
Hinman JD, Duce JA, Siman RA, Hollander W, Abraham CR. Activation of calpain-1 in myelin and microglia in the white matter of the aged rhesus monkey. J Neurochem. 2004 Apr;89(2):430-41. PubMed.
Duce JA, Hollander W, Jaffe R, Abraham CR. Activation of early components of complement targets myelin and oligodendrocytes in the aged rhesus monkey brain. Neurobiol Aging. 2006 Apr;27(4):633-44. Epub 2005 Jun 29 PubMed.
Hinman JD, Abraham CR. What's behind the decline? The role of white matter in brain aging. Neurochem Res. 2007 Dec;32(12):2023-31. Epub 2007 Apr 20 PubMed.
Hinman JD, Chen CD, Oh SY, Hollander W, Abraham CR. Age-dependent accumulation of ubiquitinated 2',3'-cyclic nucleotide 3'-phosphodiesterase in myelin lipid rafts. Glia. 2008 Jan 1;56(1):118-33. PubMed.
It would be interesting to learn if these results hold in humans. As one example: Older adults with schizophrenia are more likely to be diagnosed with dementia (Stroup et al., 2021), experience accelerated brain aging (Oct. 2019 news), and to have had defects with myelin (Sui et al., 2021).
References:
Stroup TS, Olfson M, Huang C, Wall MM, Goldberg T, Devanand DP, Gerhard T. Age-Specific Prevalence and Incidence of Dementia Diagnoses Among Older US Adults With Schizophrenia. JAMA Psychiatry. 2021 Jun 1;78(6):632-641. PubMed.
Sui YV, Bertisch H, Lee HH, Storey P, Babb JS, Goff DC, Samsonov A, Lazar M. Quantitative Macromolecular Proton Fraction Mapping Reveals Altered Cortical Myelin Profile in Schizophrenia Spectrum Disorders. Cereb Cortex Commun. 2021;2(2):tgab015. Epub 2021 Feb 24 PubMed.
Make a Comment
To make a comment you must login or register.