Fatal Gift Wrap: Neurons Fall for Packaged Tau Oligomers
Quick Links
Aggregated tau spreads through mouse brain by passing from one cell to the next, one theory goes, but how does it get in? In the November 27 Brain, researchers led by Tsuneya Ikezu at Boston University reported that tau oligomers infiltrate neurons more readily when they can hitch a ride inside extracellular vesicles. The authors isolated vesicles from Alzheimer’s and control brains, and found that only the former contained the oligomers. When injected into old wild-type mice, these AD vesicles sparked tauopathy, even though the amount of aggregated tau in the vesicles was minuscule. Injecting the same amount of free oligomeric or fibrillar tau, isolated from the same donor brains, had no effect on mouse brains. Vesicle-bound tau spreads more efficiently than free forms, Ikezu concluded.
- Extracellular vesicles from AD brains contain oligomeric tau.
- This packaged tau enters neurons more easily than does free tau.
- Vesicles may help spread tauopathy throughout the brain.
Dimitrios Kapogiannis at the National Institute on Aging, Baltimore, said this information is valuable. “This study strengthens the view that extracellular vesicles are important for AD pathogenesis, and provides strong motivation for continued research on the topic,” he wrote to Alzforum.
“Blocking the release of extracellular vesicles containing tau seeds, or blocking their uptake by unaffected neurons, may have therapeutic potential in AD,” he suggested.

Deadly Cargo. Extracellular vesicles from AD brains (right) contain more oligomeric tau (gold) than do those from prodromal AD (middle) or control (left) brains. [Courtesy of Ruan et al., Brain.]
Researchers previously implicated the smallest of extracellular vesicles, known as exosomes, in ferrying pathogenic forms of Aβ, tau, and other proteins between cells (Dec 2014 conference news). However, much of this work was done using vesicles from cultured cells or animal models. Ikezu and colleagues wanted to study whether extracellular vesicles (EVs) from human brain might be strewing pathology.
First author Zhi Ruan isolated EVs from postmortem human frontal cortices, using a protocol the group previously developed that employs sequential centrifugation and filtration steps to enrich for small EVs (Muraoka et al., 2020). Purified vesicles averaged ~150 nm in diameter, indicating they were enriched for exosomes, which range from 30 to 150 nm. However, the preparation likely contained small microvesicles, as well, which range from 100 to 1,000 nm across. The authors collected samples from three people who had had AD dementia, three who had had prodromal AD, and three cognitively healthy, age-matched controls.
Ruan found that the contents of EVs from AD, but not prodromal or control, brains bound the tau-oligomer-specific antibody TOMA (Castillo-Carranza et al., 2014). Atomic force microscopy revealed oligomers in EVs from both AD and prodromal AD brains, though the prodromal oligomers were sparse (image above). Likewise, electron microscopy detected globular tau oligomers in both AD and prodromal EVs, but not in control EVs. There was another difference as well. Aggregated tau from AD EVs resisted solubilization more than did tau from prodromal AD EVs. The authors concluded that both kinds of EV contain oligomeric tau, but AD EVs include more protofibrillar forms.
Could these vesicles seed pathology? The scientists added each type to primary mouse neurons in culture. About 40 percent of the tau from AD EVs made its way into neurons, compared to 10 percent of that in prodromal EVs. AD EVs triggered aggregation of endogenous mouse tau, as per a fluorescent FRET assay; prodromal and control EVs did not. AD EVs transfer aggregated tau and seed native tau aggregation more efficiently than do prodromal EVs, Ikezu concluded.
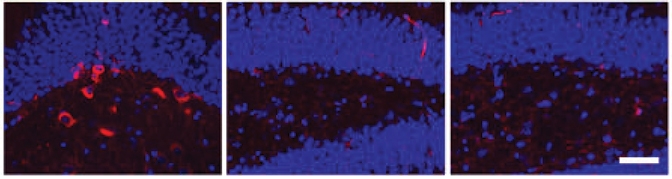
Mind the Wrap! Oligomeric tau in extracellular vesicles triggers tau phosphorylation (red) in mouse hippocampus (left), while free oligomeric tau (middle) and fibrillar tau (right) do not. Nuclei are blue. [Courtesy of Ruan et al., Brain.]
Results in mice were similar. Because females are more susceptible to tau pathology than males, the authors injected human EVs into the hippocampi of 18-month-old, female, wild-type mice. After 4.5 months, recipients of AD EVs had more phosphorylated mouse tau in their hippocampi compared to recipients of prodromal or control EVs. The authors isolated this p-tau and found it bound an antibody specific for paired helical filaments of tau and resisted solubilization. This suggests this p-tau consists of oligomeric and protofibrillar aggregates.
Notably, injected AD EVs totaled only 300 picograms of tau. Previously, much larger amounts of free tau fibrils, ranging from 1 to 8 micrograms, were needed to seed tau pathology in wild-type mice (Guo et al., 2016; Nov 2017 news). To directly compare EVs with free tau, the authors injected 300 pg of free oligomeric or fibrillar tau from the same donor brains into 18-month-old female mice. No p-tau developed after 4.5 months (image above).
“Seeding in wild-type mice has been demonstrated before, but using such a small amount, and in extracellular vesicles, is definitely new,” Lary Walker at Emory University, Atlanta, told Alzforum. He suggested that vesicular tau may be able to enter cells better than free tau because vesicles fuse with cell membranes and dump their contents inside. Because the tau concentration in AD EVs is similar to that in mouse cerebrospinal fluid, EVs may represent a more physiological paradigm of tau spread.
What kind of cells take up exosomal tau? Perhaps surprisingly, immunohistochemistry of mouse brain revealed that three-quarters of the “takers” were inhibitory interneurons, the remainder excitatory. In AD brains, most tau tangles are found in excitatory pyramidal neurons. Ikezu speculated that affected interneurons may die off quickly, and so are not visible in later stages of the disease. Interneurons are known to be more susceptible to tau toxicity than are excitatory neurons, he noted.
In mouse brains, the neurons that took up the tau vesicles became dysfunctional. Inhibitory neurons made less of the transcription factor c-fos, and formed fewer synapses with nearby excitatory neurons. In hippocampal slice cultures from these mice, excitatory pyramidal cells generated weaker spontaneous inhibitory postsynaptic currents, suggesting a failure of the inhibitory network. The pyramidal cells also fired off fewer and weaker action potentials.
Curiously, prodromal EVs sparked very little tau phosphorylation in aged mice. Even so, these vesicles dampened synaptic transmission even more than did AD EVs. “Soluble tau oligomers may induce more neurophysiological dysfunction than protofibrils,” Ikezu suggested.
In future work, Ikezu will investigate why EVs target inhibitory neurons, and what cell types produce the vesicles. EVs from AD brains express more glial markers than do those from control brains, hinting at a glial origin. Walker found this curious, because glia normally make barely any tau. Ikezu and colleagues previously demonstrated that microglia in a tauopathy mouse model can spread pathology via exosomes (Oct 2015 news), though in this study, mouse microglia did not take up the human EVs.
Ikezu also wants to find ways to block EV uptake, potentially slowing disease progression. Robert Rissman at the University of California, San Diego, agreed this will be a fruitful avenue for future research. “Knowing that tau toxicity can be propagated in this way opens the field of tauopathy therapeutics to the modulation of brain exosomes,” he wrote to Alzforum.—Madolyn Bowman Rogers
References
News Citations
- Exosomes: Purveyors of Neurodegenerative Disease?
- Human Tau Strains Propagate Faithfully in Wild-Type Mice
- Deadly Delivery: Microglia May Traffic Tau Via Exosomes
Paper Citations
- Muraoka S, Lin W, Chen M, Hersh SW, Emili A, Xia W, Ikezu T. Assessment of separation methods for extracellular vesicles from human and mouse brain tissues and human cerebrospinal fluids. Methods. 2020 May 1;177:35-49. Epub 2020 Feb 5 PubMed.
- Castillo-Carranza DL, Sengupta U, Guerrero-Muñoz MJ, Lasagna-Reeves CA, Gerson JE, Singh G, Estes DM, Barrett AD, Dineley KT, Jackson GR, Kayed R. Passive immunization with Tau oligomer monoclonal antibody reverses tauopathy phenotypes without affecting hyperphosphorylated neurofibrillary tangles. J Neurosci. 2014 Mar 19;34(12):4260-72. PubMed.
- Guo JL, Narasimhan S, Changolkar L, He Z, Stieber A, Zhang B, Gathagan RJ, Iba M, McBride JD, Trojanowski JQ, Lee VM. Unique pathological tau conformers from Alzheimer's brains transmit tau pathology in nontransgenic mice. J Exp Med. 2016 Nov 14;213(12):2635-2654. Epub 2016 Oct 17 PubMed.
Further Reading
Primary Papers
- Ruan Z, Pathak D, Venkatesan Kalavai S, Yoshii-Kitahara A, Muraoka S, Bhatt N, Takamatsu-Yukawa K, Hu J, Wang Y, Hersh S, Ericsson M, Gorantla S, Gendelman HE, Kayed R, Ikezu S, Luebke JI, Ikezu T. Alzheimer's disease brain-derived extracellular vesicles spread tau pathology in interneurons. Brain. 2021 Feb 12;144(1):288-309. PubMed. Correction.
Annotate
To make an annotation you must Login or Register.

Comments
National Institute on Aging
This study provides novel and valuable information on the role of extracellular vesicles (EVs) in the spread of pathogenic tau oligomers and propagation of tau pathology in AD.
The study’s notable strengths include a powerful demonstration that EVs contain cargo of hyperphosphorylated tau oligomers, important experimental controls (e.g., demonstration of preserved tau cargo after degradation of extravesicular proteins), and immunogold electron microscopy evidence. The data, among other implications, reinforce the view that neuronal EVs may serve as tau biomarkers for clinical and preclinical Alzheimer’s disease, something that my lab and others have been advocating for some time.
Moreover, particularly important is the evidence that EVs of Alzheimer’s patients are more capable of seeding tau pathology in old mice than are purified tau oligomers or fibrils. This suggests that blocking the release of EVs containing tau seeds or blocking their uptake by unaffected neurons may have therapeutic potential in AD.
Finally, the finding that pathogenic EVs preferentially target GABAergic neurons, at least in mice, opens avenues for future research, as it is not immediately clear which characteristics of GABAergic neurons are responsible for this preferential targeting.
Overall, this study strengthens the view that EVs are important for AD pathogenesis and provides strong motivation for continued research on the topic.
Shanghai Qiangrui Biotech Co., Ltd
DZNE (German Center for Neurodegenerative Diseases)
The Ikezu lab has published a series of pioneering papers on the role of exosomes/extracellular vesicles (EVs) in neurodegeneration, notably the role of exosomes in the spreading of tau pathology via microglia (Asai et al., 2015). The distinction between exosomes and EVs tends to be fuzzy, depending on size (exosomes are smaller, <100 nm) and other criteria. In this new study, Ruan et al. used the more general term EVs, but similar preparation as before.
They compared the physicochemical structure and pathogenic function of EVs isolated from brains of Alzheimer’s disease (AD), prodromal Alzheimer’s disease (pAD), and non-demented control cases. They found that AD EVs contained a much higher amount of epitope-specific tau oligomers (positive for tau-monomer-specific antibody TOMA1 and TOMA2, but not for TOMA3) than pAD and control EVs.
In vitro, compared with pAD and control EVs, AD EVs showed higher uptake and transfer efficiency of tau to cultured murine neurons and higher seeding activity measured by a FRET-based seeding assay. In vivo, pAD and AD EVs were more efficient in seeding and propagating tau pathology than control EVs and isolated tau oligomers and fibrils. The evidence is that the inoculation of AD or pAD EVs (containing only 300 pg of tau) into the outer molecular layer of the dentate gyrus of wild-type mice induced aggregation of endogenous tau demonstrated by the formation of sarkosyl insoluble tau, whereas inoculation of an equal amount of tau from control extracellular vesicles, isolated tau oligomers, or fibrils from the same Alzheimer’s disease donor showed little tau pathology, as judged by AT8 positive staining.
Intriguingly, pAD or AD EVs preferentially mediated tau propagation in GABAergic interneurons. They did so to a much lesser extent in excitatory mossy cells positive for glutamate receptors 2/3, leading to reduced GABAergic transmission in this region. This study raises several interesting questions.
The authors noted that inoculation of AD or pAD EVs in mouse brain induced AT8-positive tau in female mice but not in male mice, but they did not show the results and did not comment on this phenomenon. Given the different occurrence of AD between men and women, it would be interesting to test whether such EVs displayed different seeding activity between male and female mice.
References:
Arnsten AF, Datta D, Tredici KD, Braak H. Hypothesis: Tau pathology is an initiating factor in sporadic Alzheimer's disease. Alzheimers Dement. 2021 Jan;17(1):115-124. Epub 2020 Oct 19 PubMed.
Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, Wolozin B, Butovsky O, Kügler S, Ikezu T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci. 2015 Nov;18(11):1584-93. Epub 2015 Oct 5 PubMed.
Dujardin S, Bégard S, Caillierez R, Lachaud C, Delattre L, Carrier S, Loyens A, Galas MC, Bousset L, Melki R, Aurégan G, Hantraye P, Brouillet E, Buée L, Colin M. Ectosomes: a new mechanism for non-exosomal secretion of tau protein. PLoS One. 2014;9(6):e100760. Epub 2014 Jun 27 PubMed.
Kanmert D, Cantlon A, Muratore CR, Jin M, O'Malley TT, Lee G, Young-Pearse TL, Selkoe DJ, Walsh DM. C-Terminally Truncated Forms of Tau, But Not Full-Length Tau or Its C-Terminal Fragments, Are Released from Neurons Independently of Cell Death. J Neurosci. 2015 Jul 29;35(30):10851-65. PubMed.
Wang Y, Balaji V, Kaniyappan S, Krüger L, Irsen S, Tepper K, Chandupatla R, Maetzler W, Schneider A, Mandelkow E, Mandelkow EM. The release and trans-synaptic transmission of Tau via exosomes. Mol Neurodegener. 2017 Jan 13;12(1):5. PubMed.
Wischik CM, Novak M, Thøgersen HC, Edwards PC, Runswick MJ, Jakes R, Walker JE, Milstein C, Roth M, Klug A. Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4506-10. PubMed.
Wischik CM, Novak M, Thøgersen HC, Edwards PC, Runswick MJ, Jakes R, Walker JE, Milstein C, Roth M, Klug A. Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4506-10. PubMed.
Make a Comment
To make a comment you must login or register.