Does APP Promote Synapse Loss, Aβ Production Through Wnt Signaling?
Quick Links
A new signaling role is being proposed for the amyloid precursor protein. According to a September 20 paper in Translational Psychiatry, APP binds co-receptors of multiple branches of the Wingless/int-1 (Wnt) signaling pathway, variously potentiating the building and degradation of synapses. Scientists led by Richard Killick, King’s College London, report that when conditions favor the latter, neurons churn out Aβ, which tilts the scales even further toward synapse destruction. A pharmacological Wnt pathway inhibitor rescued synapses and reportedly halved the plaque burden in mice within two weeks.
- APP facilitates both canonical and noncanonical Wnt signaling.
- Aβ promotes the noncanonical path, stimulating its own release.
- Blocking this Wnt signaling cleared plaques in AD mouse models.
“This is a nice study that contributes to the discussion that ROCK [Rho-associated kinase] inhibitors, such as fasudil, could be a rational therapeutic avenue for synapse loss in Alzheimer’s disease,” said Jeremy Herskowitz, University of Alabama at Birmingham. It’s an impressive result that needs to be replicated, he added.
Nibaldo Inestrosa, who studies Wnt signaling in AD at the Pontificia Universidad Católica de Chile, Santiago, said the work was well done and revealed an interesting interplay among Wnt signaling and AD. “I encourage further studies aiming to prevent synapse loss and to improve cognition,” he wrote.
Some were more cautious and wondered if the authors had proper readouts of noncanonical Wnt signaling. Still others questioned the rapid reduction of plaques.
Feedback Loop.
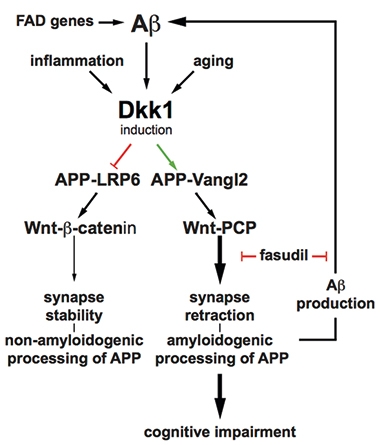
Aβ stimulates expression of Dickkopf 1, which promotes a branch of Wnt signaling that retracts synapses. The pathway also stimulates Aβ production, suggesting a self-perpetuating cycle. [Courtesy of Elliot et al., 2018.]
To dynamically regulate synapses, so-called canonical and noncanonical Wnt pathways oppose each other, either bolstering or degrading them, respectively. This balance can be scuppered by a protein called Dickkopf-1. Dkk1 is upregulated in AD postmortem brains and tilts signaling toward the noncanonical arm (e.g., Caricasole et al., 2004). Previously, Killick and other groups had reported that Aβ synapototoxicity involved expression of Dkk1 and noncanonical Wnt and could be blocked by an inhibitor of said signaling called fasudil (Killick et al., 2012; Mar 2012 news; Sellers et al., 2018).
A recent study suggested APP might complicate this scenario. Researchers found that the fruit fly homologue of APP bound co-receptors for Wnt signaling, potentiating the noncanonical path (Soldano et al., 2013). In the current paper, first author Christina Elliot and colleagues explored the possibility that APP modulates Wnt signaling to affect synaptic stability.
The researchers found that in rat primary cortical neurons, APP bound the transmembrane proteins LRP6 and Vangl2, co-activators of the canonical and noncanonical Wnt pathways, respectively. APP potentiated both types of signaling. In HEK293 cells, the Swedish mutation in APP, which causes autosomal-dominant AD, preferentially activated noncanonical Wnt signaling and inhibited the canonical pathway. The authors wrote that this result suggested a direct effect of the mutation on synapses.
Elliot and colleagues also report that activators of canonical Wnt signaling reduce Aβ production in HEK293 cells, while activators of noncanonical signaling have the opposite effect, increasing Aβ production. Also, HEK293 cells expressing APPSwe released more Aβ into culture medium than cells expressing the wild-type protein. All told, the data paint noncanonical Wnt signaling as promoting Aβ production.
The results hint at an Aβ feedback loop. Since the peptide promotes Dkk1 expression, and Dkk1 ultimately raises Aβ, then Aβ may stimulate its own production through noncanonical Wnt signaling, the authors suggest.

Fasudil Foils Plaques.
After two weeks on fasudil, 18-month-old 3xTg mice had half as many Aβ plaques as their untreated littermates. [Courtesy of Elliot et al., 2018.]
Could blocking noncanonical Wnt signaling interrupt this cycle? The authors treated primary rat cortical neurons with fasudil, which triggers noncanonical Wnt signaling and reportedly rescues tau pathology in fruit flies (Feb 2016 news). As the authors had seen previously, adding Dkk1 to the neuron depleted dendritic spines, whereas fasudil prevented this. The drug also prevented an uptick in Aβ production. In 18-month-old 3xTg mice—four male and three female—which had already accumulated Aβ plaques, two weeks of fasudil injections reportedly halved soluble brain Aβ and plaques relative to four male and six female mice treated with a placebo (see image at right).
“They show categorically that fasudil can clear up the plaques; that’s an amazing result,” said Patricia Salinas, University College London. It would have been helpful to see if synapse loss had also slowed in the treated animals, she added. “This is a new interesting target that should be considered [for Alzheimer’s disease].” Salinas cautioned that fasudil inhibits a number of other kinases besides ROCK, so future experiments should clarify if fasudil clears plaques by blocking noncanonical Wnt signaling or by some other means. Figuring out how it works may help design even more effective drugs, she said.
A long list of drugs have been reported over the years to have cleared up plaques in 3xTg mice, highlighting the limitations of predicting efficacy in human AD based on transgenic APP/PS mouse models. Even so, some scientists were puzzled that fasudil would have cleared brain plaques so fast. “It is surprising to see a claim of rapid plaque disappearance with an inhibitor of Wnt signaling, which is theoretically directed at reducing Aβ production,” said Greg Cole, University of California, Los Angeles. Cole thinks that a small sample size and more females than males in the control group could have skewed the results, because female 3xTg mice reportedly deposit more plaques than males (Carroll et al., 2010).
“I understand that fadusil may be neuroprotective and block some Aβ effects or production, but don’t grasp how it would be rapidly promoting clearance,” Cole wrote. “Nonetheless, even if the apparent rapid plaque disappearance doesn’t hold up, the other protective effects are still encouraging, because fadusil is approved for clinical use in Japan,” he added.—Gwyneth Dickey Zakaib
References
News Citations
Mutations Citations
Research Models Citations
Paper Citations
- Caricasole A, Copani A, Caraci F, Aronica E, Rozemuller AJ, Caruso A, Storto M, Gaviraghi G, Terstappen GC, Nicoletti F. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in Alzheimer's brain. J Neurosci. 2004 Jun 30;24(26):6021-7. PubMed.
- Killick R, Ribe EM, Al-Shawi R, Malik B, Hooper C, Fernandes C, Dobson R, Nolan PM, Lourdusamy A, Furney S, Lin K, Breen G, Wroe R, To AW, Leroy K, Causevic M, Usardi A, Robinson M, Noble W, Williamson R, Lunnon K, Kellie S, Reynolds CH, Bazenet C, Hodges A, Brion JP, Stephenson J, Paul Simons J, Lovestone S. Clusterin regulates β-amyloid toxicity via Dickkopf-1-driven induction of the wnt-PCP-JNK pathway. Mol Psychiatry. 2012 Nov 20; PubMed.
- Sellers KJ, Elliott C, Jackson J, Ghosh A, Ribe E, Rojo AI, Jarosz-Griffiths HH, Watson IA, Xia W, Semenov M, Morin P, Hooper NM, Porter R, Preston J, Al-Shawi R, Baillie G, Lovestone S, Cuadrado A, Harte M, Simons P, Srivastava DP, Killick R. Amyloid β synaptotoxicity is Wnt-PCP dependent and blocked by fasudil. Alzheimers Dement. 2018 Mar;14(3):306-317. Epub 2017 Oct 19 PubMed.
- Soldano A, Okray Z, Janovska P, Tmejová K, Reynaud E, Claeys A, Yan J, Atak ZK, De Strooper B, Dura JM, Bryja V, Hassan BA. The Drosophila homologue of the amyloid precursor protein is a conserved modulator of Wnt PCP signaling. PLoS Biol. 2013 May;11(5):e1001562. PubMed.
- Carroll JC, Rosario ER, Kreimer S, Villamagna A, Gentzschein E, Stanczyk FZ, Pike CJ. Sex differences in β-amyloid accumulation in 3xTg-AD mice: role of neonatal sex steroid hormone exposure. Brain Res. 2010 Dec 17;1366:233-45. PubMed.
Further Reading
Papers
- Sellers KJ, Elliott C, Jackson J, Ghosh A, Ribe E, Rojo AI, Jarosz-Griffiths HH, Watson IA, Xia W, Semenov M, Morin P, Hooper NM, Porter R, Preston J, Al-Shawi R, Baillie G, Lovestone S, Cuadrado A, Harte M, Simons P, Srivastava DP, Killick R. Amyloid β synaptotoxicity is Wnt-PCP dependent and blocked by fasudil. Alzheimers Dement. 2018 Mar;14(3):306-317. Epub 2017 Oct 19 PubMed.
- Marzo A, Galli S, Lopes D, McLeod F, Podpolny M, Segovia-Roldan M, Ciani L, Purro S, Cacucci F, Gibb A, Salinas PC. Reversal of Synapse Degeneration by Restoring Wnt Signaling in the Adult Hippocampus. Curr Biol. 2016 Oct 10;26(19):2551-2561. Epub 2016 Sep 1 PubMed.
Primary Papers
- Elliott C, Rojo AI, Ribe E, Broadstock M, Xia W, Morin P, Semenov M, Baillie G, Cuadrado A, Al-Shawi R, Ballard CG, Simons P, Killick R. A role for APP in Wnt signalling links synapse loss with β-amyloid production. Transl Psychiatry. 2018 Sep 20;8(1):179. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Imperial College London
The observation by Elliot et al. that the Wnt-PCP pathway affects the processing of APP is original and interesting. However, we already published in 2015 that DKK1 increases the generation of amyloid-β by affecting the transcription of BACE1 (Parr et al., 2015). In fact, we reported that β-catenin binds specifically to regions within the promoter of BACE1 containing putative T-cell factor/lymphoid enhancer binding factor-1 (TCF/LEF) motifs, consistent with canonical Wnt target regulation. Therefore, we proposed a different mechanism of action that does not involve the regulation of the non-amyloidogenic processing of APP.
References:
Parr C, Mirzaei N, Christian M, Sastre M. Activation of the Wnt/β-catenin pathway represses the transcription of the β-amyloid precursor protein cleaving enzyme (BACE1) via binding of T-cell factor-4 to BACE1 promoter. FASEB J. 2015 Feb;29(2):623-35. Epub 2014 Nov 10 PubMed.
Make a Comment
To make a comment you must login or register.