Death of the Neatnik: Neurons Perish When Trash Clutters Their Space?
Quick Links
Perhaps nerve cells are simply neurotic about neatness. Perhaps they keel over when they get overloaded with junk? This view of Alzheimer disease draws support from two new papers highlighting the importance in AD of autophagy, a means by which cells dispose of unwanted proteins. In a study published online June 10 in Cell, New York University researchers with collaborators elsewhere report that the AD-linked protein presenilin-1 (PS1) is needed for lysosomal degradation during autophagy, and that pathogenic PS1 mutations stymie the process. The study identifies a novel function for PS1 that is critical for neuronal survival and does not necessarily depend on Aβ or amyloid, said principal investigator Ralph Nixon of the NYU-affiliated Nathan Kline Institute for Psychiatric Research. Instead, it has to do, surprisingly, with glycosylation of a proton pump. This study found its autophagy tie-in by examining neurons that lack, or have mutations in, an AD-related gene. The second study, published June 15 in PLoS ONE, also connects autophagy and AD but does so by looking at how deficiency of an autophagy regulator worsens AD pathology. In that study, Tony Wyss-Coray of Stanford University, Palo Alto, California, and colleagues report that beclin-1 regulates turnover of amyloid precursor protein (APP), the source of the toxic Aβ peptides littering AD brains.
Prior immunolabeling and electron microscopy studies by the Nixon lab suggested that autophagy revs up in AD (Nixon et al., 2005) and leads to increased Aβ production (Yu et al., 2005). The researchers made the leap to look at presenilins based on immunocytochemistry studies that revealed full-length PS1 and PS2 in the endoplasmic reticulum (Zhang et al., 1998), where the proteins have been proposed to serve as ion channels (Tu et al., 2006 and ARF related news story). Figuring the ER was important for formation of autophagosomes during autophagy, “we had a hunch that if PS1 were involved at all in the lysosomal system, it might be doing something in the ER, and that pathogenic PS1 mutations might somehow enhance the disturbance in the lysosomal system,” Nixon told ARF. Earlier work from his lab showed enhanced lysosomal pathology in neurons with familial AD-linked PS1 mutations, compared to cells from sporadic AD cases (Cataldo et al., 2004). “That established the pathological basis for then going back into the cells and asking what happens if we knock out presenilin-1?” Nixon said.
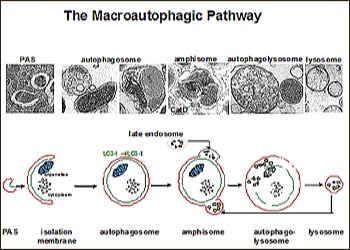
Using metabolic labeling methods to analyze blastocysts from wild-type or PS1 knockout mice, first author Ju-Hyun Lee and colleagues found that PS1-deficient cells had decreased autophagic turnover of long-lived proteins. The defect was specific, as synthesis and degradation of short-lived proteins seemed fine without PS1. Ultrastructural analyses confirmed that autophagy had gone awry in PS1-deficient cells. Similar to their previous analyses of autopsy tissue from AD patients, PS1-deficient neurons were stalled in the earlier stages of autophagy—i.e., crammed with more autophagosomes and early autolysosomes—whereas most of the intermediate autophagic vesicles in wild-type cells were late autolysosomes. From there, the researchers worked backward, systematically examining step after step in the autophagy process, to figure out what was causing autophagy to lag in the absence of PS1.
View larger image Image credit: Ralph Nixon
The early steps of autophagy looked normal in the PS1 knockout cells. “We could write off everything that is involved in the signaling and in the formation of the autophagosome, and even in the fusion with the lysosome. That simplified our task,” Nixon said. Focusing then on what happens after autophagosome and lysosome have fused, the researchers did several critical experiments. First, using dyes that selectively immunolabel the active form of cathepsins D and B, they saw that the lysosomal proteases were present but largely inactive in PS1-deficient cells. Second, their studies with LysoTracker, an indicator that lights up in acidic compartments, pointed the finger at pH as the culprit for the proteolysis defects in PS1-deficient cells. When LysoTracker did not fluoresce in structures clearly identified as lysosomes based on presence of cathepsins or other markers, the only explanation was that the pH is off without PS1, Nixon said. The scientists saw these same defects in fibroblasts from AD patients with pathogenic PS1 mutations. Furthermore, they found support for their hunch about the pH problem when they were able to make wild-type cells look just as autophagy-challenged as PS1-knockout cells by neutralizing the pH with ammonium chloride or bafilomycin A1.
Bafilomycin A1 is an inhibitor of the proton pump (v-ATPase) that acidifies the lysosome. And as luck would have it, the proton pump was the scientists’ first guess as to what might be going wrong in the absence of PS1, Nixon said. Immunostaining PS1 knockout cells, he and colleagues saw that the bulk of v-ATPase had not yet made it into lysosomes but was still in the ER. Subfractionation experiments verified the ER localization and yielded another important piece of the puzzle: the ER v-ATPase was unglycosylated, whereas most of the v-ATPase in wild-type cells occurs in glycosylated form largely in lysosomes. The suspicion that cells without PS1 were failing to glycosylate v-ATPase gained strength when biochemical experiments showed PS1 co-immunoprecipitating with all the relevant players to glycosylate v-ATPase. “When we found that part of the complex included the oligosaccharyl transferase, the mechanism was clear,” Nixon said. “That complex mediates glycosylation of the v-ATPase, which is required for its delivery to the lysosome. Without PS1, the v-ATPase remains unglycosylated and stuck in the ER.”
Presenilin aficionados may find it intriguing that the glycosylation complex contained full-length PS1—not the cleaved form found in γ-secretase, the enzymatic complex that helps cut Aβ out of APP. But the biggest news to the AD field is that the PS1 mutations “directly affect a function that is critical for cell survival—namely, lysosomal function,” Nixon said. “That in itself, regardless of whatever else Aβ may be doing, may be enough to account for the profound increase in aggressiveness of PS forms of familial AD compared with sporadic forms.”
The findings offer a fresh look at the autophagy/lysosomal process, whereas previous studies have primarily connected these pathways with γ-secretase or Aβ. For example, in the April issue of the journal Autophagy, researchers led by Toshiyuki Nakagawa, Gifu University School of Medicine, Japan, propose that the autophagy-lysosomal system modulates γ-secretase activity through GCN2 (general control nonderepressible 2), and that cells lacking Atg5 (a gene in the autophagy pathway) rack up more Aβ (Ohta et al., 2010). The data seem consistent with prior studies showing increased neurodegeneration in Atg5 knockout mice (Hara et al., 2006 and ARF related news story). The authors and others believe the new studies should stir interest in targeting autophagic/lysosomal pathways for AD therapeutics (see Jaeger, Masliah, and Nakagawa comments below).
Recent work has fingered another autophagy regulator, beclin-1, in worsening of AD pathology. Wyss-Coray and colleagues reported in 2008 that beclin-1 levels are reduced in AD brains, and that AD transgenic mice on a beclin-1 heterozygote background had more Aβ deposition and synaptic abnormalities than AD mice with wild-type beclin-1 (Pickford et al., 2008 and ARF related news story). That study “made a link among AD, amyloidosis, and autophagy. We now provide a cell biology rationale as to why that might be the case,” Wyss-Coray said of the work his team reports today in PLoS ONE.
In the current study, first author Philipp Jaeger and colleagues knocked down beclin-1 in cell culture, and as a consequence, detected more full-length APP, APP C-terminal fragments, and extracellular Aβ. “You basically get accumulation of all APP fragments, which strongly suggests APP is targeted to the autophagosomal pathway,” Wyss-Coray said. In line with this, lentiviral overexpression of beclin-1 reduced APP levels in transduced CHO/hAPP cells.
In frontal cortex samples from AD patients, relative to age-matched healthy tissue, the researchers found that lower beclin-1 levels corresponded to a similar drop in the protein VPS34 (aka PIK3C3, for class 3 phosphoinositide-3-kinase). VPS34 along with beclin-1 and other proteins form a complex that initiates autophagy. In the same AD brains, the researchers found that levels of the autophagy protein Atg5 were unchanged, suggesting that only parts of the autophagy pathway are dysregulated in AD.
All told, the Stanford group provides evidence to suggest beclin-1 deficiency may not only keep the autophagy process from getting started, but also block later degradation of autophagosomes. “This is where our two stories come together,” Wyss-Coray said, referring to his and Nixon’s studies (see full comment below). “We basically get the same phenotype as you get with PS1 deficiency or mutations. There is a stall somewhere in the autophagosomes’ fusion with lysosomes, such that APP is hanging around in the cell much longer and cannot be degraded.”
This could result from problems when autophagosomes form, which happens early in the autophagy process with the help of beclin-1. However, the stalling could also reflect interference with beclin-dependent late endosome functions. Disturbances at this step are known to disrupt the forming of amphisomes (i.e., fusion of autophagosome and endosome) and their clearance through autophagy (Filimonenko et al., 2007), Nixon noted (see full comment below).—Esther Landhuis
References
News Citations
- Presenilins Open Escape Hatch for ER Calcium
- Autophagy Prevents Inclusions, Neurodegeneration
- Autophagy Regulator Helps Neurons Stomach Excess Aβ, Resist AD
Paper Citations
- Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005 Feb;64(2):113-22. PubMed.
- Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH, Mohan PS, Mercken M, Farmery MR, Tjernberg LO, Jiang Y, Duff K, Uchiyama Y, Näslund J, Mathews PM, Cataldo AM, Nixon RA. Macroautophagy--a novel Beta-amyloid peptide-generating pathway activated in Alzheimer's disease. J Cell Biol. 2005 Oct 10;171(1):87-98. PubMed.
- Zhang J, Kang DE, Xia W, Okochi M, Mori H, Selkoe DJ, Koo EH. Subcellular distribution and turnover of presenilins in transfected cells. J Biol Chem. 1998 May 15;273(20):12436-42. PubMed.
- Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell. 2006 Sep 8;126(5):981-93. PubMed.
- Cataldo AM, Peterhoff CM, Schmidt SD, Terio NB, Duff K, Beard M, Mathews PM, Nixon RA. Presenilin mutations in familial Alzheimer disease and transgenic mouse models accelerate neuronal lysosomal pathology. J Neuropathol Exp Neurol. 2004 Aug;63(8):821-30. PubMed.
- Ohta K, Mizuno A, Ueda M, Li S, Suzuki Y, Hida Y, Hayakawa-Yano Y, Itoh M, Ohta E, Kobori M, Nakagawa T. Autophagy impairment stimulates PS1 expression and gamma-secretase activity. Autophagy. 2010 Apr;6(3):345-52. PubMed.
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006 Jun 15;441(7095):885-9. PubMed.
- Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, Wyss-Coray T. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008 Jun;118(6):2190-9. PubMed.
- Filimonenko M, Stuffers S, Raiborg C, Yamamoto A, Malerød L, Fisher EM, Isaacs A, Brech A, Stenmark H, Simonsen A. Functional multivesicular bodies are required for autophagic clearance of protein aggregates associated with neurodegenerative disease. J Cell Biol. 2007 Nov 5;179(3):485-500. PubMed.
Other Citations
Further Reading
Papers
- Ohta K, Mizuno A, Ueda M, Li S, Suzuki Y, Hida Y, Hayakawa-Yano Y, Itoh M, Ohta E, Kobori M, Nakagawa T. Autophagy impairment stimulates PS1 expression and gamma-secretase activity. Autophagy. 2010 Apr;6(3):345-52. PubMed.
- Yu WH, Cuervo AM, Kumar A, Peterhoff CM, Schmidt SD, Lee JH, Mohan PS, Mercken M, Farmery MR, Tjernberg LO, Jiang Y, Duff K, Uchiyama Y, Näslund J, Mathews PM, Cataldo AM, Nixon RA. Macroautophagy--a novel Beta-amyloid peptide-generating pathway activated in Alzheimer's disease. J Cell Biol. 2005 Oct 10;171(1):87-98. PubMed.
- Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005 Feb;64(2):113-22. PubMed.
- Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, Wyss-Coray T. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008 Jun;118(6):2190-9. PubMed.
Primary Papers
- Jaeger PA, Pickford F, Sun CH, Lucin KM, Masliah E, Wyss-Coray T. Regulation of amyloid precursor protein processing by the Beclin 1 complex. PLoS One. 2010;5(6):e11102. PubMed.
- Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, Wolfe DM, Martinez-Vicente M, Massey AC, Sovak G, Uchiyama Y, Westaway D, Cuervo AM, Nixon RA. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010 Jun 25;141(7):1146-58. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of California, San Diego
In this study, Lee and colleagues describe a novel function of presenilin-1 (PS1), a protein previously found and best characterized as being involved in γ-secretase cleavage of amyloid precursor protein (APP) and Notch. The authors report that PS1 knockout cells exhibit a marked reduction in autolysosomal protein degradation in response to autophagy activation induced by serum starvation. On the subcellular level, these PS1 knockout cells present a phenotype that resembles histopathological changes in Alzheimer disease brains: the accumulation of numerous membrane-bound vesicles of the autolysosomal pathway (autophagosomes, early and late autolysosomes) that are filled with amorphous, undigested, electron-dense material.
Furthermore, the authors provide evidence for impaired maturation of cathepsin D, an important lysosomal protease, in the PS1 knockout cells. They show that this deficiency is due to reduced acidification of the lysosomal lumen. In a comprehensive attempt to identify the underlying mechanistic defects, Lee and colleagues discovered the involvement of PS1 in the maturation and localization of v-ATPase V0a1. This proton pump is important to establish a low intra-lysosomal pH. PS1 directly binds to the ATPase, modulates its glycosylation state, and in this way modifies the ATPase's maturation, degradation, and subcellular localization. The authors conclude that PS1 knockout causes decreased levels of mature v-ATPase V0a1, which in turn leads to impaired lysosomal acidification and decreased lysosomal proteolysis. They continue to support this hypothesis with data from PS1 hypomorphic mice and from AD patients' fibroblasts. In the hypomorphic mice PS1 levels are high enough to sustain Notch cleavage and prevent developmental defects, but appear insufficient to maintain normal lysosomal protein turnover. In the AD fibroblasts, certain PS1 mutations seem to strongly inhibit proteolysis, indicating that these mutations likely play a role in PS1-v-ATPase V0a1 interactions. Lee and colleagues thus propose a novel function of PS1 in lysosomal acidification, based on v-ATPase V0a1 maturation, which could be contributing to the observed accumulation of aberrant autophagosomes and lysosomes in AD patients’ brain tissue.
View all comments by Philipp JaegerThe recent report from Randy Nixon and colleagues is an interesting development in the story of familial Alzheimer disease (FAD) and its molecular roots. It is established that autophagy is deficient in the neurons of Alzheimer disease patients and that increased or induced autophagy can reverse these deficits. However, until now, the underlying mechanism of the deficient autophagy has not been clear. Nixon and colleagues have identified a defect in the acidification of the lysosome organelle specifically associated with mutations in PS1 found in FAD. While PS1 mutations have long been associated with increases in Aβ, this paper identifies a function for the holoprotein as a chaperone in the ER. Furthermore, the researchers were able to identify the ATPase complex that is dissociated in PS1 mutants. These important findings could lead to new avenues of therapies that target the ATPase complex by targeting the chaperone function of PS1.
View all comments by Eliezer Masliah�
Nixon and colleagues show the involvement of PS1 in autophagy/lysosomal function, and indicate that PS1 mutations in familial AD cause its impairment.
View all comments by Toshiyuki NakagawaWe believe that the autophagic/lysosomal pathway is a key therapeutic target, and it is important to investigate further if improving its function would be beneficial for decreasing amyloid-β production in vivo.
New York University School of Medicine/Nathan Kline Institute
The results in Jaeger et al. reinforce previous work from their lab (Pickford et al., 2008) showing that autophagy is a significant APP turnover pathway that can be strongly upregulated in cells. During autophagy induction, APP levels decreased somewhat more than Aβ levels, consistent with earlier data that Aβ is generated during autophagy and that efficient lysosomal proteolysis is needed to prevent the intracellular buildup of Aβ in autophagic vacuoles and lysosomes seen in AD. The authors also show that the block in autophagosome clearance in AD and AD mouse models can be exacerbated by beclin deficiency. This may be due to the impairment of early autophagy steps of autophagosome formation, which require beclin, as proposed in the paper, and it could also reflect interference with beclin-dependent late endosome functions, disturbances of which are known to disrupt amphisome formation and clearance through autophagy (1). Interestingly, the resultant buildup of APP and β-CTF in endosomal-related compartments is reminiscent of the conditions that develop in Down syndrome, where it has been shown that APP duplication, through elevations of β-CTF, accelerates endocytosis (2). This creates more substrate traffic to lysosomes, interferes with endosome functions (2,3), and we suspect, also impairs autophagic turnover. These new investigations add to mounting evidence that disruption of lysosomal clearance mechanisms may be a critical factor in AD pathogenesis.
References:
Filimonenko M, Stuffers S, Raiborg C, Yamamoto A, Malerød L, Fisher EM, Isaacs A, Brech A, Stenmark H, Simonsen A. Functional multivesicular bodies are required for autophagic clearance of protein aggregates associated with neurodegenerative disease. J Cell Biol. 2007 Nov 5;179(3):485-500. PubMed.
Jiang Y, Mullaney KA, Peterhoff CM, Che S, Schmidt SD, Boyer-Boiteau A, Ginsberg SD, Cataldo AM, Mathews PM, Nixon RA. Alzheimer's-related endosome dysfunction in Down syndrome is Abeta-independent but requires APP and is reversed by BACE-1 inhibition. Proc Natl Acad Sci U S A. 2010 Jan 26;107(4):1630-5. Epub 2009 Dec 28 PubMed.
Cataldo AM, Mathews PM, Boiteau AB, Hassinger LC, Peterhoff CM, Jiang Y, Mullaney K, Neve RL, Gruenberg J, Nixon RA. Down syndrome fibroblast model of Alzheimer-related endosome pathology: accelerated endocytosis promotes late endocytic defects. Am J Pathol. 2008 Aug;173(2):370-84. PubMed.
Stanford University Medical School
The study by Nixon and colleagues is an absolutely gorgeous paper. It is cell biology at its best. It is interesting that our studies manipulating beclin-1 arrive at a very similar pathology as the lack of presenilin, both in vivo and in cell culture, causing an abnormal accumulation of lysosomes and autolysosomes (see also Pickford et al., 2008). Indeed, beclin-1 may have a role not only in the initiation of autophagy, but as an increasing number of studies suggest, in vesicle trafficking as well. Whether beclin-1 and presenilins interact at some level will be interesting to explore in the future.
However, we could have a friendly debate about whether autophagy really requires presenilin as stated in the title, since the study does not actually manipulate the autophagy process. Rather, it interferes with the final degradative step and shows very nicely that presenilins are necessary for lysosomal degradation.
If autophagy is the process of manufacturing garbage bags, filling them with trash, and hauling them to the dump, lysosomal degradation would be the incineration of the trash-filled bags. For the incinerator to run, you need fuel and pipes that bring the fuel into the incinerator (the ATPase). If the incinerator does not run, you will naturally accumulate the bags filled with trash, but it would not follow that garbage bag production is impaired.
Furthermore, it is likely that endosomal trafficking and multivesicular bodies are equally affected by lysosomal degradation, but the paper does not discuss these possibilities.
References:
Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, Wyss-Coray T. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008 Jun;118(6):2190-9. PubMed.
View all comments by Tony Wyss-CorayNew York University School of Medicine/Nathan Kline Institute
Reply to comment by Tony Wyss-Coray
Since its very early descriptions, autophagy has been defined as the lysosomal digestion of a cell’s own cytoplasmic material and not simply the sequestration of these components. Implied by this definition, and generally accepted in the autophagy field, is the central concept that lysosomal proteolysis is required to complete autophagy. This is a critical point, especially because autophagy failure in disease states, as measured by the diminished turnover of specific autophagy substrates, can result from failure of substrate sequestration, autophagosome formation, fusion of autophagosomes with a lysosome, or digestion of the substrate. Distinguishing which step in autophagy is defective in different neurodegenerative diseases has become important and usually involves evaluating not only autophagosome formation, but also autophagic flux, which reflects the balance between substrate sequestration and proteolytic clearance (1). Narrowly defining autophagy as only the sequestration step during autophagy, as proposed by Tony Wyss-Coray, does not recognize the well-accepted key role of the lysosome in this self-digestion process.
Tony points out that our results imply, but do not show, that cargoes delivered to lysosomes by endocytosis should also be affected by lysosomal failure. Indeed, we present data in our paper demonstrating that lysosomal turnover of endosomal substrates is decreased, as one might predict. Thus, in addition to being required for autophagy, presenilin-1 is required for the turnover of endosomal substrates.
References:
Wong E, Cuervo AM. Autophagy gone awry in neurodegenerative diseases. Nat Neurosci. 2010 Jul;13(7):805-11. PubMed.
View all comments by Ralph NixonMake a Comment
To make a comment you must login or register.