Bexarotene—First Clinical Results Highlight Contradictions
Quick Links
The cancer medication bexarotene, aka Targretin®, made headlines with a report that it quickly lowered brain Aβ, reduced plaque load, and improved behavioral deficits in mouse models of Alzheimer’s disease. Now, the first clinical results are coming in, and they hint that the Aβ effect may not translate to people—especially if they carry an ApoE4 allele.
In the January 29 Alzheimer’s Research and Therapy, scientists led by Jeffrey Cummings, Cleveland Clinic Lou Ruvo Center for Brain Health, Las Vegas, report that in a small, double-blind, placebo-controlled trial, four weeks of bexarotene treatment did not reduce brain amyloid as seen on PET scans in patients with AD. However, florbetapir imaging did suggest that the drug slightly lowered amyloid in a subset of people—those who did not carry the ApoE4 genetic risk factor for AD. The authors propose that these results lay the groundwork for a larger Phase 2 trial.
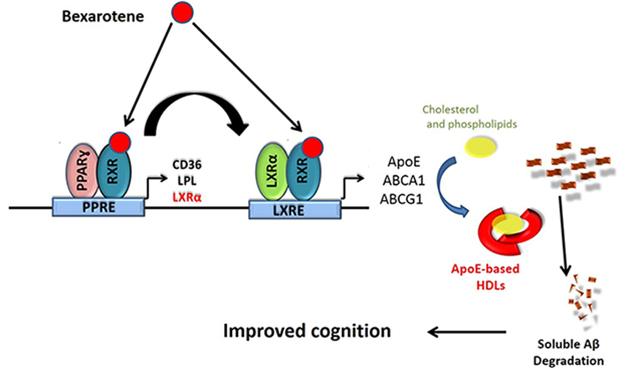
Basis for Bexarotene. Could the drug help AD patients by promoting clearance of Aβ via ApoE? [Courtesy of Gary Landreth.]
Some independent support for this conclusion comes from a case report in Belgium, though not necessarily because of Aβ reduction. Over 20 months, a man with early AD was reportedly stable while on the drug. And yet, PET and cerebrospinal fluid (CSF) analysis indicated little change in his brain Aβ levels. Therefore, his clinicians, Adrian Ivanoiu and colleagues at the Université catholique de Louvain, Brussels, concluded that his apparent cognitive benefit may have occurred independently of amyloid reduction (Pierrot et al., 2015).
While a single case supports no formal conclusions, unpublished results from a third source, a pilot CSF trial, seem to point in the same direction. This study was co-led by Gary Landreth, Case Western Reserve University in Cleveland, who was the principal investigator on the original mouse study that spurred interest in bexarotene for AD. Landreth told Alzforum that while the drug increased brain ApoE levels in non ApoE4 carriers, it did not change soluble Aβ over the short term.
The upshot of all this research is that researchers are at odds over what bexarotene does in the brain and whether further trials are warranted. Landreth, who co-founded ReXceptor Inc. to study bexarotene and other retinoid X receptor (RXR) agonists in neurodegenerative diseases, believes that the Lou Ruvo study provides a rationale for a larger trial, but he warned against overenthusiasm. “We shouldn’t get ahead of ourselves, as this was a small study from which no hard conclusions can be drawn,” he told Alzforum. Other scientists urged caution because of concerns about cardiovascular risk; bexarotene is known to increase triglycerides and cholesterol in the blood.
Cummings and colleagues strongly warned against using bexarotene off-label because of its cardiovascular risks. Other medications can bring down levels of cholesterol and triglycerides, but a longer observation is needed to see if they can keep them down, Cummings told Alzforum. Complicating the issue further, recent evidence suggests that statins lower ABCA1 expression, meaning they could counteract the effects of bexarotene (Niesor et al., 2015).
Bexarotene binds RXRs, which activate multiple genes (see graphic above). Some of those control cell differentiation and proliferation, hence its use for tumor regression. RXRs also activate transcription of ApoE and the ATP-binding cassette A1 (ABCA1), which helps load lipids onto the apolipoprotein. Scientists believe that lipidated ApoE helps remove Aβ from the brain. Recent work suggest the drug directly interferes with Aβ fibrillization (see Part 2 of this story).
At Lou Ruvo, Cummings and colleagues designed the Phase 2a BEAT-AD trial as a proof-of-concept study, to see whether bexarotene would lower amyloid burden as estimated by way of florbetapir PET. The researchers recruited 20 volunteers aged 50 to 90 who had brain amyloid, a diagnosis of AD according to the NINCDS-ADRDA criteria, and MMSE scores between 10 and 20 (McKhann et al., 1984). Sixteen patients received 75 mg of bexarotene twice a day for the first seven days, then double that for the next three weeks; four got placebo. In a four-week, open-label extension trial, all patients received the drug. The trial monitored patients for elevations in plasma cholesterol and triglycerides, side effects of bexarotene treatment. Where necessary, the researchers controlled cholesterol with atorvastatin and triglycerides with clofibrate.
As the primary outcome, the researchers measured change from baseline to week four in plaque burden as measured by the standard uptake value ratio (SUVR) of florbetapir using white matter as a reference region (Chen et al., 2015). The authors prespecified that they would analyze changes based on ApoE4 status, though genotypes remained blinded until the end of the trial. Changes in scores on the MMSE, ADAS-Cog, and other neuropsychological tests were secondary outcomes. The researchers also measured serum levels of Aβ42 and Aβ40.
In the overall group, PET imaging indicated that bexarotene failed to lower plaque burden. Cognition did not change in the 16 treated patients either, though there was an uptick in their serum Aβ42. Cholesterol levels rose in 11 treated people and triglycerides in 15—in one person more than 10-fold. Treatment did not cause amyloid-related imaging abnormalities (ARIA).
In four treated patients who had no ApoE4 allele, uptake of florbetapir was lower at week four than at baseline in the anterior and posterior cingulate, parietal and temporal cortices, and precuneus. A composite SUVR based on these five regions and the frontal medial orbital fell by about 0.145 relative to change in placebo group. The increase in serum Aβ42 in people on the drug correlated with the reduction in their florbetapir uptake, suggesting that soluble amyloid was being transferred from the brain to the blood. While the trial was small and the treatment period short, the authors concluded that bexarotene may reduce plaque, at least in ApoE4 non-carriers.
Data on the four-week, open-label extension and changes in biomarkers are forthcoming, Cummings told Alzforum. He plans to design a Phase 2/3 trial in patients with mild to moderate AD, both ApoE4 carriers and non-carriers. To capture any clinical benefits, the trial should last at least 12 months, he said.
Why would the drug benefit only ApoE4 non-carriers? The authors noted that plaques in ApoE4 carriers are more compact, making them harder to solubilize. In addition, ApoE4 is harder to lipidate than ApoE2 or ApoE3. On the other hand, Cummings suggested that it just may take more time to see benefits in carriers.
Plaques Persist.
Bexarotene slightly reduced flutemetamol signal in this AD patient. [Courtesy Adrian Ivanoiu and Journal of Alzheimer’s Disease.]
For the Belgian patient, who was heterozygous for ApoE4, no strong evidence emerged for plaque reduction over six months. This 68-year-old man, who had an MMSE of 26, was diagnosed with Alzheimer’s based on cognitive testing, FDG PET, and flutemetamol PET scans for Aβ. He requested bexarotene upon learning about Landreth’s mouse study. After consulting their local ethics committee, his physicians agreed. They prescribed 300mg bexarotene per day, along with 200mg fenofibrate to limit any increase in plasma cholesterol and triglycerides. After six months he underwent renewed cognitive testing, a second lumbar puncture for fluid biomarker analysis, and another amyloid PET scan. His memory, as judged by free and cued recall tests, improved by 40 percent and he scored 28 on the MMSE. His CSF total tau and phospho-tau fell by 20 and 10 percent, respectively, while CSF Aβ nudged up by 10 percent. The man’s six-month amyloid PET SUVR registered at 1.94, slightly below his baseline value of 2.08. Total plasma cholesterol stayed below 250mg/dl. First author Nathalie Pierrot and colleagues concluded that the memory and tau effects may be unrelated to amyloid changes, which they considered to be within test-retest variation.
That’s the data published so far. Ivanoiu further told Alzforum that the man continued on bexarotene for a total of 23 months. Twenty months after beginning the treatment, his MMSE was only slightly below his baseline score of 26. Alas, the patient could not afford the monthly cost of bexarotene—about €1,200—and stopped taking it. His cognition quickly worsened, Ivanoiu said. MMSE scores at three, five, and eight months after stopping the drug were 24, 23, and 20, respectively. While there’s no way to tell if this was a placebo effect, Ivanoiu and colleagues noted that the approved AD drug galantamine had no effect on his memory when he took it after his initial diagnosis. “Bexarotene is no miracle drug; nevertheless, I think some effect was present,” Ivanoiu told Alzforum. He hopes his findings will spur further research.
Landreth’s own Phase 1b study, conducted in collaboration with C2N Diagnostics, also suggests bexarotene does not shift Aβ in the cerebrospinal fluid in people. This trial measured the acute effects of bexarotene on the synthesis and clearance of both ApoE and Aβ in in healthy, young adult ApoE4 non-carriers. Landreth told Alzforum that while bexarotene does not easily cross the blood-brain barrier, over five days it raised the level of ApoE in CSF by about 25 percent. There was no change in CSF Aβ during that time, however, which is in stark contrast to what he had reported in mice. Landreth had shown that bexarotene reduced soluble Aβ in mouse models of AD within hours and halved plaque load within three days (Feb 2012 news story). Other researchers who attempted to replicate these mouse results reported no change in plaques, but some found that the drug reduced soluble Aβ in the mouse brain and improved learning and memory (May 2013 news). Researchers at Amgen recently attempted to repeat this again in rats, reporting that neither bexarotene nor the LXR agonist TO901317 altered CSF Aβ40. Their paper awaits peer review on F1000Research. Landreth contends that failures to replicate his original data can be attributed to the use of unconventional preparations of the drug, rather than the microcrystalline formulation used in the clinic (summarized by Tesseur et al., 2013).
What are researchers to make of these latest reports? Other scientists were skeptical that bexarotene will work for Alzheimer’s. “The results of this [Lou Ruvo] study are quite clear and do not support the use of bexarotene for the treatment of AD,” wrote Danny Michaelson, Tel Aviv University. Mary Jo LaDu and Leon Tai at the University of Illinois at Chicago added that they do not think another Phase 2 trial is justified. Cummings said it was unthinkable not to investigate this drug further, given the needs of the patients and the failure rate of other disease-modifying agents. “I think we can be a little less tentative since this is the first small-molecule agent ever to show amyloid lowering in a double-blind placebo-controlled study,” he told Alzforum.
LaDu and Tai cautioned that the cardiovascular effects make bexarotene too risky for Alzheimer’s patients and hypothesized that microbleeds in the brain would become a problem with prolonged use. They and Michaelson argued for testing a safer drug that hits the ABCA1 target more cleanly. Landreth noted that the clinical literature suggests medications effectively control dyslipidemia in cancer patients taking bexarotene, but he hopes that pharmaceutical companies will design a similar molecule that better enters the CNS and has little effect on plasma cholesterol and triglycerides.—Gwyneth Dickey Zakaib and Tom Fagan
References
Therapeutics Citations
News Citations
- Bexarotene in AD—New Twist to its M.O.
- Upping Brain ApoE, Drug Treats Alzheimer's Mice
- Bexarotene Revisited: Improves Mouse Memory But No Effect on Plaques
Paper Citations
- Pierrot N, Lhommel R, Quenon L, Hanseeuw B, Dricot L, Sindic C, Maloteaux JM, Octavea JN, Ivanoiu A. Targretin Improves Cognitive and Biological Markers in a Patient with Alzheimer's Disease. J Alzheimers Dis. 2015;49(2):271-6. PubMed.
- Niesor EJ, Schwartz GG, Perez A, Stauffer A, Durrwell A, Bucklar-Suchankova G, Benghozi R, Abt M, Kallend D. Statin-induced decrease in ATP-binding cassette transporter A1 expression via microRNA33 induction may counteract cholesterol efflux to high-density lipoprotein. Cardiovasc Drugs Ther. 2015 Feb;29(1):7-14. PubMed.
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984 Jul;34(7):939-44. PubMed.
- Chen K, Roontiva A, Thiyyagura P, Lee W, Liu X, Ayutyanont N, Protas H, Luo JL, Bauer R, Reschke C, Bandy D, Koeppe RA, Fleisher AS, Caselli RJ, Landau S, Jagust WJ, Weiner MW, Reiman EM, Alzheimer’s Disease Neuroimaging Initiative. Improved power for characterizing longitudinal amyloid-β PET changes and evaluating amyloid-modifying treatments with a cerebral white matter reference region. J Nucl Med. 2015 Apr;56(4):560-6. Epub 2015 Mar 5 PubMed.
- Tesseur I, De Strooper B. When the dust settles: what did we learn from the bexarotene discussion?. Alzheimers Res Ther. 2013;5(6):54. Epub 2013 Nov 7 PubMed.
External Citations
Further Reading
Papers
- Boehm-Cagan A, Michaelson DM. Reversal of apoE4-driven brain pathology and behavioral deficits by bexarotene. J Neurosci. 2014 May 21;34(21):7293-301. PubMed.
- Riancho J, Berciano MT, Berciano J, Lafarga M. Relaunching an old drug: the potential role of bexarotene in neurodegenerative diseases. J Neurol. 2016 Jan;263(1):177-8. Epub 2016 Jan 2 PubMed.
- Tachibana M, Shinohara M, Yamazaki Y, Liu CC, Rogers J, Bu G, Kanekiyo T. Rescuing effects of RXR agonist bexarotene on aging-related synapse loss depend on neuronal LRP1. Exp Neurol. 2015 Dec 11;277:1-9. PubMed.
- Tousi B. The emerging role of bexarotene in the treatment of Alzheimer's disease: current evidence. Neuropsychiatr Dis Treat. 2015;11:311-5. Epub 2015 Feb 5 PubMed.
Primary Papers
- Cummings JL, Zhong K, Kinney JW, Heaney C, Moll-Tudla J, Joshi A, Pontecorvo M, Devous M, Tang A, Bena J. Double-blind, placebo-controlled, proof-of-concept trial of bexarotene Xin moderate Alzheimer's disease. Alzheimers Res Ther. 2016 Jan 29;8:4. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Illinois, Chicago
University of Illinois
Four years after the controversial bexarotene publication from the Landreth group, Cummings and colleagues publish this clinical trial. This proof of concept (POC) trial tested bexarotene (300 mg/kg) in 16 mild to moderate AD patients versus placebo controls. The authors found no efficacy in their primary objective—evaluating amyloid burden as measured by florbetapir PET at baseline and following four weeks of treatment in treated versus placebo controls, neither stratified by APOE4 status. There was efficacy in reducing amyloid burden for the APOE4 non-carriers (n=4) versus placebo (n=3). However, the small sample size limits the overall impact. Additionally, in the APOE4 non-carrier group only, increased efficacy (reduced amyloid signal) correlated with increased serum cholesterol and triglyceride, highlighting safety concerns and the potential benefits of a tissue selective nuclear receptor agonist. The findings confirm ApoE as a therapeutic target for AD. Unfortunately, instead of clarifying the effects of this drug on AD pathology, the results seem to complicate the understanding of the mechanisms of the disease and contribute to the consensus that bexarotene is not a drug for AD.
A lack of efficacy cannot be explained by the short treatment duration because, overall, reduction of cortical amyloid burden correlated with increased cholesterol and triglycerides (due to activation of RXR receptors in the liver), and decreased cognition. Furthermore, even in APOE4 non-carriers, the safety of long-term bexarotene is questionable, given that the side effects are known to increase AD risk, potentially nullifying any benefits. Lastly, as oligomeric Aβ is likely the proximal neurotoxin in AD, mobilization of Aβ from plaques may increase the levels of soluble Aβ in brain, possibly increasing synapto-toxicity and explaining the reduction in cognition.
This study also adds to the controversy regarding the role of ApoE in Aβ clearance, because bexarotene apparently has a different effect in AD patients than in familial AD-transgenic (FAD-Tg) mice. In the latter, it decreases soluble Aβ but not insoluble Aβ levels, but only in FAD-Tg APOE4 carriers. In contrast, this very limited human data suggests treatment is effective only in APOE4 non-carriers.
Although the authors justify the number of participants as a POC study, there were not enough APOE4 carrier placebo subjects for a proper statistical comparison. The “mean” for APOE4 carriers in the placebo group was based on one person. The authors report that the study was not powered to detect differences in cognition, but they also report a significant p-value indicating that cognition is compromised by bexarotene treatment (or that it improved in placebo). It is not clear why this readout is included if the trial was insufficiently powered to detect an effect. Relevant data such as APOE genotype stratified by sex and baseline characteristics, as well as a clear definition of safety parameters, would have been helpful.
Lack of amyloid burden excluded 16 potential participants. Importantly, because positive APOE4 status correlates with increased amyloid burden, basing inclusion on a positive amyloid scan likely biased the sample to APOE4 carriers.
University of Nevada Las Vegas
A few points are worth making:
Proof-of-mechanism (POM) studies are by nature exploratory and are intended only to decide if further exploration of an agent or mechanism is warranted. Many current agents in advanced trials have not shown that they affect the target biology in early POM studies. To my knowledge only one other agent currently at the human level of investigation—aducanumab—has shown reduction of fibrillar amyloid in a double-blind randomized trial. Soluble amyloid is reduced in CSF by BACE inhibitors, providing increased confidence that these agents are affecting the desired target.
The observations in our study suggest that bexarotene affects both soluble and insoluble amyloid. The preliminary effects of bexarotene suggest that bexarotene or other retinoid-X receptor agonists should be investigated in Alzheimer's disease. We eagerly anticipate further studies to determine if the initial observations with bexarotene are confirmed.
Make a Comment
To make a comment you must login or register.