Aβ Fibrils Drive Oligomer Formation, New Model Suggests
Quick Links
As attention shifts toward free-floating Aβ oligomers as the prime molecular culprits in Alzheimer’s disease, there is still plenty of confusion over the role of fibrillar amyloid, the stuff that sits in the disease’s hallmark brain plaques. Now, researchers report that Aβ fibrils act as nucleation centers, speeding the assembly of monomers into toxic oligomers. In the new model, monomers alone can still clump to form oligomers, but this is not the rate-limiting step, said Christopher Dobson, University of Cambridge, U.K., a corresponding author on the study published online May 23 in the Proceedings of the National Academy of Sciences USA. “The rate-limiting step is the secondary nucleation process involving fibril-catalyzed aggregation,” Dobson said. Tuomas Knowles of the University of Cambridge and Sara Linse of Lund University in Sweden are the study’s other corresponding authors.
“This is one of the finest papers I have ever read. It is unbelievably rigorous work,” said David Teplow, who studies the biophysics of Aβ aggregation at the University of California, Los Angeles. Other scientists also found the data quality and analysis impressive, though some wondered if the secondary nucleation pathway would be relevant to human AD and whether it could influence current therapeutic approaches.
Even as Aβ oligomers garnered increasing attention in AD research, the process by which these toxic assemblies form and grow remained unclear. The kinetic data varied considerably, leading scientists to assume amyloid aggregation was a random process. However, several years ago Linse and colleagues made key improvements in Aβ peptide purification and solvent preparation that enabled them to measure the fibrillization dynamics of human Aβ42 produced and purified from bacteria in a reproducible way (Hellstrand et al., 2010). Those data suggested that Aβ fibrils do not form randomly, but rather through a predictable sequence of events. Meanwhile, Dobson said, Knowles and colleagues reported a “mathematical breakthrough”—a new way to use kinetic equations to simultaneously evaluate the contribution of multiple factors thought to be important for aggregation (Knowles et al., 2009). “Our motivation was to take Linse’s quality experimental data and extract rate constants in the individual steps of fibril formation,” Dobson said.
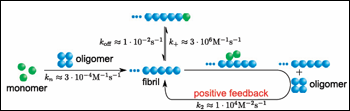
In the current study, first author Samuel Cohen and colleagues considered three possible models for Aβ42 aggregation and tested them against experimentally measured aggregation. In one model, the rate of Aβ42 aggregation depends only on monomer concentration. However, fibril stability can also influence aggregation. If Aβ assemblies grow primarily at their ends, then fragmentation would be a major driver, as breaking fibrils apart produces more ends. The second model reflected that scenario, with the formation of Aβ aggregates depending strictly on fibril concentration. The researchers also considered a third possibility—secondary nucleation—in which the surfaces of fibrils catalyze formation of entirely new aggregates at a rate that depends on the concentration of both monomers and fibrils (see image below).
Does the Alzheimer's brain know this much math? A new model proposes that Aβ oligomers form through secondary nucleation on existing fibrils. Image courtesy of PNAS and Tuomas Knowles
Tested with 10 initial monomer concentrations, the third model gave the best fit for the experimental data. This suggests that once fibrils form, they catalyze formation of new oligomers. If that were indeed the dominant mechanism driving production of the toxic species, then the model would predict faster oligomerization of Aβ42 monomers in the presence of preformed fibrils. Sure enough, this is what the researchers saw, but they were still unsure this was due to secondary nucleation. They needed to confirm the source of the oligomers—to distinguish, for instance, whether the oligomers were breaking off from existing fibrils or newly forming from monomers.
The scientists did two radiolabelling experiments to discern these possibilities. In one, they mixed unlabeled fibrils into a solution of radiolabelled Aβ42 monomers and saw that this produced radiolabelled oligomers, suggesting that the oligomers arose from the monomers. In the reverse experiment—addition of labeled fibrils to unlabeled Aβ42 monomers—the resulting oligomers had no radioactivity, confirming that they did not originate from the preformed fibrils. Together, these data “show that the Aβ42 oligomers are coming from the monomeric species through fibril-catalyzed secondary nucleation,” Dobson said.
Do oligomers that form this way in a test tube look anything like the ones that have been isolated from human AD brain, and do they behave similarly? At this point, Cohen and colleagues know little about the structure of the oligomers formed by secondary nucleation, but they have begun testing their biological activity. The researchers prepared two solutions—one containing Aβ42 monomers mixed with preformed fibrils to drive secondary nucleation, the other containing just Aβ42 monomers. They purified the oligomers produced in each aggregation reaction and put them into cultures of SH-SY5Y human neuroblastoma cells. Oligomers from the fibril-spiked monomer solution drove up caspase 3/7 activity and killed more cells than did oligomers from monomers only, linking secondary nucleation with oligomer toxicity in cell culture.
While the current data suggest fibrils are important for perpetuating the production of toxic Aβ oligomers, other recent work provides direct evidence for this secondary nucleation phenomenon. In a paper posted online February 13 in the Journal of Molecular Biology, researchers led by Giovanni Dietler and Hilal Lashuel at the Swiss Federal Institute of Technology (EPFL), Lausanne, report using atomic force microscopy to visualize new aggregates forming on the surface of Aβ42 fibrils (Jeong et al., 2013). Previous in-vitro studies by Lashuel and others had hinted that secondary nucleation was important for assembly of pathological Aβ species (Jan et al., 2011; Jan et al., 2008; Wogulis et al., 2005), and the current findings build on that work.
Furthermore, Knowles’ new model seems consistent with prior in-vivo data showing Aβ oligomers surrounding plaques in AD transgenic mice and AD patients (see ARF related news story on Koffie et al., 2009), said Tara Spires-Jones of Massachusetts General Hospital in Boston. The current results “suggest that Aβ monomers interact with fibrils in the plaques to make new oligomers, and that may explain why we see oligomers spinning around the plaques,” Spires-Jones told Alzforum.
How is it that amyloid fibrils might catalyze oligomer formation? “We think the surface of fibrils may be quite attractive to monomers,” Dobson said. The fibrils’ “stickiness” thus raises the local concentration of monomers, increasing their chances of glomming together, he suggested.
If this fibril-driven aggregation is, in fact, the rate-limiting step of Aβ aggregation, as the new model would predict, then therapeutic approaches aimed at stopping primary nucleation may not be effective, Dobson said. He said his team is looking for molecules that target the secondary pathway by binding amyloid fibrils or inhibiting their interaction with monomers.
However, Brigita Urbanc of Drexel University in Philadelphia, Pennsylvania, noted that the key pathological processes in AD are widely believed to occur prior to fibril formation—in which case, blocking the secondary nucleation mechanism may not do much good. “It would likely affect processes further removed from the initial triggers of disease,” she said.
Dominic Walsh of Brigham and Women’s Hospital, Boston, Massachusetts, also wondered how relevant the current findings would be to human AD. “Translating the lessons learned from this carefully controlled model to the chaos of the diseased brain will be challenging, not least because the brain has multiple Aβ peptides of different primary sequence and many surfaces on which to interact,” he wrote in an e-mail to Alzforum.
In a similar vein, the rate-limiting steps for proliferation of α-synuclein, tau, and other toxic molecular assemblies “could be quite varied in different diseases,” Dobson said.—Esther Landhuis
References
News Citations
Paper Citations
- Hellstrand E, Boland B, Walsh DM, Linse S. Amyloid β-protein aggregation produces highly reproducible kinetic data and occurs by a two-phase process. ACS Chem Neurosci. 2010 Jan 20;1(1):13-8. PubMed.
- Knowles TP, Waudby CA, Devlin GL, Cohen SI, Aguzzi A, Vendruscolo M, Terentjev EM, Welland ME, Dobson CM. An analytical solution to the kinetics of breakable filament assembly. Science. 2009 Dec 11;326(5959):1533-7. PubMed.
- Jeong JS, Ansaloni A, Mezzenga R, Lashuel HA, Dietler G. Novel Mechanistic Insight into the Molecular Basis of Amyloid Polymorphism and Secondary Nucleation during Amyloid Formation. J Mol Biol. 2013 May 27;425(10):1765-81. PubMed.
- Jan A, Adolfsson O, Allaman I, Buccarello AL, Magistretti PJ, Pfeifer A, Muhs A, Lashuel HA. Abeta42 neurotoxicity is mediated by ongoing nucleated polymerization process rather than by discrete Abeta42 species. J Biol Chem. 2011 Mar 11;286(10):8585-96. PubMed.
- Jan A, Gokce O, Luthi-Carter R, Lashuel HA. The ratio of monomeric to aggregated forms of Abeta40 and Abeta42 is an important determinant of amyloid-beta aggregation, fibrillogenesis, and toxicity. J Biol Chem. 2008 Oct 17;283(42):28176-89. PubMed.
- Wogulis M, Wright S, Cunningham D, Chilcote T, Powell K, Rydel RE. Nucleation-dependent polymerization is an essential component of amyloid-mediated neuronal cell death. J Neurosci. 2005 Feb 2;25(5):1071-80. PubMed.
- Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, Micheva KD, Smith SJ, Kim ML, Lee VM, Hyman BT, Spires-Jones TL. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci U S A. 2009 Mar 10;106(10):4012-7. PubMed.
Other Citations
Further Reading
Papers
- Jeong JS, Ansaloni A, Mezzenga R, Lashuel HA, Dietler G. Novel Mechanistic Insight into the Molecular Basis of Amyloid Polymorphism and Secondary Nucleation during Amyloid Formation. J Mol Biol. 2013 May 27;425(10):1765-81. PubMed.
- Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, Micheva KD, Smith SJ, Kim ML, Lee VM, Hyman BT, Spires-Jones TL. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci U S A. 2009 Mar 10;106(10):4012-7. PubMed.
- Jan A, Adolfsson O, Allaman I, Buccarello AL, Magistretti PJ, Pfeifer A, Muhs A, Lashuel HA. Abeta42 neurotoxicity is mediated by ongoing nucleated polymerization process rather than by discrete Abeta42 species. J Biol Chem. 2011 Mar 11;286(10):8585-96. PubMed.
- Jan A, Gokce O, Luthi-Carter R, Lashuel HA. The ratio of monomeric to aggregated forms of Abeta40 and Abeta42 is an important determinant of amyloid-beta aggregation, fibrillogenesis, and toxicity. J Biol Chem. 2008 Oct 17;283(42):28176-89. PubMed.
- Wogulis M, Wright S, Cunningham D, Chilcote T, Powell K, Rydel RE. Nucleation-dependent polymerization is an essential component of amyloid-mediated neuronal cell death. J Neurosci. 2005 Feb 2;25(5):1071-80. PubMed.
Primary Papers
- Cohen SI, Linse S, Luheshi LM, Hellstrand E, White DA, Rajah L, Otzen DE, Vendruscolo M, Dobson CM, Knowles TP. Proliferation of amyloid-β42 aggregates occurs through a secondary nucleation mechanism. Proc Natl Acad Sci U S A. 2013 Jun 11;110(24):9758-63. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
EPFL/ND BioSciences
Recent studies from a joint collaboration between our group and the Dietler laboratory at the EPFL provide, for the first time, direct evidence that Aβ42 fibrillization occurs via a combined mechanism of nucleated polymerization and secondary nucleation (see Jeong et al., 2013).
However, our AFM studies demonstrated that secondary nucleation occurs on the surface of only specific types of Aβ fibrils. For example, in the case of Aβ42, only "type 2" fibrils appear to be capable of growing via secondary nucleation events.
We did not investigate the generation of oligomers as a result of secondary nucleation events on the surface of fibrils, but the data provided by Knowles and colleagues provide strong evidence that this is possible.
It would be interesting to determine how the structural morphology of the parent fibrils influences the frequency and rate of secondary nucleation and what molecular factors and/or cellular events may influence this process.
More importantly, the results presented by Knowles and colleagues provide very nice mechanistic explanations for recent experimental observations from our group, suggesting that the process of nucleated polymerization plays a critical role in regulating Aβ toxicity. In this work, we showed that Aβ solutions containing a mixture of monomeric and aggregated Aβ are more toxic than solutions containing purely aggregated forms (fibrils or oligomers). We demonstrated a direct correlation between toxicity and the ability of Aβ species to form fibrils, and that this process depends on monomeric Aβ. Reintroduction of monomeric Aβ into nontoxic solutions containing Aβ protofibrils was sufficient to restore both toxicity and amyloid formation, whereas selective removal of monomeric Aβ from a toxic Aβ mixture (containing monomeric and aggregated Aβ species) was sufficient to block fibril formation.
We had postulated that fibrils associate with cellular membranes and that toxicity may be the result of 1) fibril-catalyzed formation of toxic Aβ species; 2) disruption of membranes as a result of the fibril formation process; or 3) activation of specific cellular pathways. The results reported by Knowles et al. provide evidence in support of the first, though we did not consider the possibility of secondary nucleation in this process (see Jan et al., 2011).
However, it is important to stress that Aβ toxicity may occur via multiple mechanisms involving the formation of specific Aβ aggregates or the process of Aβ fibril formation. There is no reason to think that there is a single toxic species or mechanism that is responsible for Aβ-induced neurodegeneration in AD.
References:
Jeong JS, Ansaloni A, Mezzenga R, Lashuel HA, Dietler G. Novel Mechanistic Insight into the Molecular Basis of Amyloid Polymorphism and Secondary Nucleation during Amyloid Formation. J Mol Biol. 2013 May 27;425(10):1765-81. PubMed.
Jan A, Adolfsson O, Allaman I, Buccarello AL, Magistretti PJ, Pfeifer A, Muhs A, Lashuel HA. Abeta42 neurotoxicity is mediated by ongoing nucleated polymerization process rather than by discrete Abeta42 species. J Biol Chem. 2011 Mar 11;286(10):8585-96. PubMed.
Brigham & Women's Hospital
This is a very nice study that brings together the use of several different approaches—mathematical modeling of protein polymerization, reliable assay systems to measure kinetics of Aβ aggregation, and innovative use of radiolabeled Aβ and size-exclusion chromatography. The conclusions reached build on a range of earlier findings including prior in-vitro data that also hinted at the importance of secondary nucleation (e.g., Wogulis et al., 2005; Jan et al., 2008; Jeong et al., 2013), and speculation that interaction between plaques (fibrils) and soluble Aβ is critical for toxicity (Koffie et al., 2009; Spires et al., 2009; McDonald et al., 2010; Esparaza et al., 2012). The most impressive aspects of this study are the quality of the kinetic data and the rigor of the analyses, both of which indicate that amyloid fibrils provide a catalytic surface for the generation of Aβ oligomers.
After thoughtfully considering the major factors believed to be important in Aβ aggregation (monomer concentration, nucleus formation, fibril elongation, and fibril fragmentation), the authors generated three predictive models and then tested them versus experimentally determined data. Thus, the outcome largely hinged on the use of a highly reproducible assay to monitor aggregation (Hellstrand et al., 2009). By examining the aggregation process under different shear conditions, they demonstrated that fragmentation of fibrils (as predicted) can also influence aggregation kinetics. Then, by seeding radiolabeled Aβ monomer with preformed unlabeled Aβ fibrils, they found that fibrils can catalyze the formation of oligomers, but that “ThT transparent” oligomers never account for more than 1 percent of the total mass of Aβ present. From Figure 4B, these oligomers appear to elute in or near to the void of a Superdex 75 column and therefore could be consistent with polydispersed prefibrillar assemblies such as protofibrils (Walsh et al., 1999; Harper et al., 1999) or ADDLs (Lambert et al., 1998; Hepler et al., 2006).
Finally, the authors present data suggesting that oligomers are biologically active. Although this is what one would expect, these experiments are the least solid part of the paper. Since it is not clear if these oligomers are stable during prolonged incubation and the toxicity assays were measured over 24 hours, it will be important to characterize oligomer activity using more rapid (and disease-relevant) assays. It will be similarly important to gain information about the structure of these oligomers and how they compare with assemblies isolated from human brain. Translating the lessons learned from this carefully controlled model to the chaos of the diseased brain will be challenging, not least because in the brain there are multiple Aβ peptides of different primary sequence and an abundance of surfaces on which to interact. Nonetheless, as an old-fashioned reductionist, I’m hopeful this study will help us inch a little closer to the worthy goal of preventing the formation (or neutralizing the effects) of toxic assemblies of Aβ.
References:
Wogulis M, Wright S, Cunningham D, Chilcote T, Powell K, Rydel RE. Nucleation-dependent polymerization is an essential component of amyloid-mediated neuronal cell death. J Neurosci. 2005 Feb 2;25(5):1071-80. PubMed.
Jan A, Gokce O, Luthi-Carter R, Lashuel HA. The ratio of monomeric to aggregated forms of Abeta40 and Abeta42 is an important determinant of amyloid-beta aggregation, fibrillogenesis, and toxicity. J Biol Chem. 2008 Oct 17;283(42):28176-89. PubMed.
Jeong JS, Ansaloni A, Mezzenga R, Lashuel HA, Dietler G. Novel Mechanistic Insight into the Molecular Basis of Amyloid Polymorphism and Secondary Nucleation during Amyloid Formation. J Mol Biol. 2013 May 27;425(10):1765-81. PubMed.
Koffie RM, Meyer-Luehmann M, Hashimoto T, Adams KW, Mielke ML, Garcia-Alloza M, Micheva KD, Smith SJ, Kim ML, Lee VM, Hyman BT, Spires-Jones TL. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci U S A. 2009 Mar 10;106(10):4012-7. PubMed.
Spires-Jones TL, Mielke ML, Rozkalne A, Meyer-Luehmann M, de Calignon A, Bacskai BJ, Schenk D, Hyman BT. Passive immunotherapy rapidly increases structural plasticity in a mouse model of Alzheimer disease. Neurobiol Dis. 2009 Feb;33(2):213-20. PubMed.
Mc Donald JM, Savva GM, Brayne C, Welzel AT, Forster G, Shankar GM, Selkoe DJ, Ince PG, Walsh DM, . The presence of sodium dodecyl sulphate-stable Abeta dimers is strongly associated with Alzheimer-type dementia. Brain. 2010 May;133(Pt 5):1328-41. PubMed.
Esparza TJ, Zhao H, Cirrito JR, Cairns NJ, Bateman RJ, Holtzman DM, Brody DL. Amyloid-β oligomerization in Alzheimer dementia versus high-pathology controls. Ann Neurol. 2013 Jan;73(1):104-19. PubMed.
Hellstrand E, Boland B, Walsh DM, Linse S. Amyloid β-protein aggregation produces highly reproducible kinetic data and occurs by a two-phase process. ACS Chem Neurosci. 2010 Jan 20;1(1):13-8. PubMed.
Walsh DM, Hartley DM, Kusumoto Y, Fezoui Y, Condron MM, Lomakin A, Benedek GB, Selkoe DJ, Teplow DB. Amyloid beta-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J Biol Chem. 1999 Sep 3;274(36):25945-52. PubMed.
Harper JD, Wong SS, Lieber CM, Lansbury PT. Assembly of A beta amyloid protofibrils: an in vitro model for a possible early event in Alzheimer's disease. Biochemistry. 1999 Jul 13;38(28):8972-80. PubMed.
Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998 May 26;95(11):6448-53. PubMed.
Hepler RW, Grimm KM, Nahas DD, Breese R, Dodson EC, Acton P, Keller PM, Yeager M, Wang H, Shughrue P, Kinney G, Joyce JG. Solution state characterization of amyloid beta-derived diffusible ligands. Biochemistry. 2006 Dec 26;45(51):15157-67. PubMed.
View all comments by Dominic WalshMake a Comment
To make a comment you must login or register.