ApoE4 Hastens Alzheimer’s Disease in Down’s Syndrome
Quick Links
ApoE4 accelerates Alzheimer’s disease in sporadic and familial cases, but does it do the same in people with Down’s syndrome? In the July 6 JAMA Neurology, researchers led by Juan Fortea and Alexandre Bejanin at the Hospital of Sant Pau in Spain reported that indeed, it does. Compared to noncarriers of this lipoprotein risk allele, cognition faltered an average of two years earlier in adults with DS and ApoE4. Their cortical metabolism slowed, hippocampi shrank, plaque loads grew, cerebrospinal fluid Aβ1-42/1-40 levels fell, and plasma phospho-tau181 levels rose earlier. "As a sporadic AD researcher, I was surprised that ApoE4 can still have an effect, despite the amyloid saturation seen in people with DS," Bejanin told Alzforum.
- In people with Down’s and ApoE4, cortical metabolism slowed, hippocampi shrank …
- … plaque load and plasma p-tau181 rose, CSF Aβ42/40 fell earlier.
- CSF p-tau181, total tau, and both fluid NfL levels were no different in this study.
In contrast, CSF ptau-181, CSF total tau, and fluid neurofilament light (NfL) did not differ between carriers and noncarriers. While both the authors and commentators were perplexed by that, they chalk up this discrepancy to having fewer CSF samples. “Logically, we believe the p-tau we see in plasma comes from the CSF, so if we see it in the plasma, we should see it in the CSF,” Fortea said. “If we had tau PET, I suspect we could have seen differences in tangles,” he added.
As for the lack of an NfL signal, Cynthia Lemere, Harvard Medical School, Boston; David Holtzman, Washington University School of Medicine, St. Louis; and Elizabeth Head, University of California, Irvine, suggest in an accompanying JAMA editorial that APOE influences dementia onset independently of NfL. “Not seeing differences in tau and NfL markers could mean they operate differently than through APOE, which predominantly affects the amyloid pathway,” Head told Alzforum.
Research on Alzheimer’s disease in Down’s syndrome has made great strides in the past decade, from building longitudinal studies and analyzing biomarker trajectories to assembling trial-ready cohorts in preparation for testing AD treatments in DS (May 2021 news). Last year, Fortea and colleagues charted the natural history course of DS, showing that markers shift in similar ways in familial AD and AD-DS, but at a younger age in the latter (Fortea et al., 2020). Adults with Down's and ApoE4 previously were reported to show dementia symptoms some two years earlier than noncarriers (Prasher et al., 2008). Thus, the scientists wondered if APOE genotype influences the speed and severity of biomarker changes as well.
To find out, co-first authors Bejanin, Maria Florencia Iulita, and colleagues gathered cognitive scores, imaging scans, fluid samples, and APOE genotype from 464 adults with DS in the Cambridge Dementia in Downs Syndrome (DiDS) cohort in the U.K., and in the Downs Alzheimer Barcelona Neuroimaging Initiative (DABNI) cohort in Spain. Of those, 354 had plasma and 158 had CSF samples, 175 had structural MRI and 132 had FDG PET scans, and 75 had amyloid PET scans. Most participants were in their mid-30s to 50 years old. Twenty-one percent carried at least one copy of ApoE4, four had both.
Overall, the number of participants with symptomatic AD was similar across ApoE genotypes. However, differences emerged when the researchers broke participants into five-year age groups. Symptomatic AD was 3.3 times more prevalent in 40- to 45-year-old carriers than in noncarriers. Likewise, carriers were more likely to be diagnosed with AD symptoms at a younger age (see image below). Fortea and colleagues found E4 hastened the appearance of symptoms—diagnosis at 51 years compared to 53 years in noncarriers—just as other researchers had found previously. None of the 97 ApoE4 carriers lived to be more than 60 years old, whereas 18 of the 367 noncarriers were 60 or older.
William Mobley, University of California, San Diego, was surprised that dementia diagnosis was delayed in noncarriers by only two years, given that the difference amounts to a decade in the general population (Corder et al., 1993). “Why is there such a modest E4 effect in people with DS? Does E4 work differently in them? Is so much Aβ made in them that it overwhelms E4’s ability to impact how much is cleared or not?” Mobley asked.
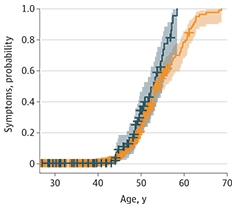
Sped Up. ApoE4 carriers (teal) develop AD symptoms at a younger age than noncarriers (orange). [Courtesy of Bejanin et al., JAMA Neurology, 2021.]
To learn whether APOE genotype affects cognition, the scientists used two tests adapted for people with DS: the Cambridge Cognitive Examination for Older Adults with Down Syndrome (CAMCOG-DS) for global cognition, and the modified Cued Recall Test (mCRT) for episodic memory. After grouping participants by genotype, Bejanin, Iulita, and colleagues saw no significant difference in CAMCOG-DS scores. However, beginning around age 40, immediate and delayed recall worsened faster in ApoE4 carriers than in noncarriers.
Neuroimaging corroborated this. Again starting around age 40, carriers lost more hippocampal gray matter than did noncarriers. Glucose metabolism slowed earlier in carriers’ occipital and parietal cortices, subcortical structures, and posterior insula. Amyloid PET revealed earlier and greater plaque deposition in carriers, beginning in their mid-30s.
Were these changes reflected in fluid biomarkers? In CSF from ApoE4 carriers, the Aβ42/40 ratio was lower than in noncarriers beginning in the 20s; however, it later converged with that of noncarriers as their Aβ42 levels began dropping around age 40. “This is due to floor effects of the assay seen in people with DS,” Fortea commented.
Other markers were less clear. While carriers had more plasma p-tau181 than noncarriers starting in their 40s, CSF p-tau181, total tau, and both fluid measures of NfL were the same across genotypes (see image below).

Genotype Matters, For Some. Compared to cognitively normal general population controls (dashed gray lines) and DS ApoE4 noncarriers (orange), AD biomarkers turned abnormal earlier in DS ApoE4 carriers (teal). They had more plaques (top left), smaller hippocampi (top middle), slower brain glucose metabolism (top right), lower CSF Aβ1-40/Aβ1-42 ratios (middle left), and more plasma ptau-181 (bottom left). CSF ptau-181 (middle middle), NfL (middle right), and plasma NfL (bottom middle) were not different. [Courtesy of Bejanin et al., JAMA Neurology, 2021.]
This lack of a signal stands in contrast to previously identified AD-related differences in plasma p-tau181 and plasma NfL in 366 participants from the DABNI cohort and 44 controls (Nov 2020 news). Now published in the July 15 Nature Communications, Fortea and colleagues reported that plasma p-tau181 and NfL identified AD dementia with AUCs of 0.94 and 0.96, respectively. P-tau181 also tracked with temporoparietal atrophy and hypometabolism. Fortea said the team is currently studying p-tau217 and 231.
What about ApoE2—did it stave off decline? Only 12 percent of participants carried at least one copy of this allele. “It was not a completely beautiful story because we did not have the statistical power to assess ApoE2 carriers, but there was a protective trend,” Fortea said. Michael Rafii, University of Southern California, Los Angeles, was also curious about E2’s effect, noting the limited evidence in DS (Royston et al., 1996). Bejanin wondered about a dosing effect of two E4 alleles versus one.
To answer those lingering questions, the authors want to analyze a larger cohort, perhaps combining data from the U.S. and U.K.-based Alzheimer's Biomarkers Consortium–Down Syndrome (ABC-DS). Head and Beau Ances, Washington University School of Medicine, St. Louis, said their groups are measuring the effects of ApoE4 on imaging, CSF, and plasma biomarkers in ABC-DS. “Does E4 shift markers earlier in our cohort, as well?” Ances wondered. “I’m hoping we will be able to replicate and extend these observations and see how all the results converge,” Head said.
Therapeutic Potential
The overall similarity between sporadic AD and AD-DS begs the question of whether anti-amyloid therapies may help people with DS. AC Immune recently reported that its anti-amyloid vaccine appears safe and induced anti-amyloid antibodies in DS (May 2021 news). Aduhelm (aducanumab) is now approved in the U.S.
A group of AD-DS researchers recently issued a consensus statement urging inclusion of DS participants in trials testing Aduhelm. For its part, the LuMind IDSC Foundation, a DS advocacy group, published an article on the drug’s potential use in DS. It draws attention to safety concerns, as microhemorrhages are more common in people with DS. Head and Fortea agreed, also noting the higher prevalence of cerebral amyloid angiopathy in DS. “We have to be cautious about safety, but that doesn’t mean we should not be ambitious and test aducanumab,” he said.
Rafii and the editorial authors highlighted potential benefits of ApoE-targeting therapies in DS, which have been shown to clear plaques in two different mouse models of amyloidosis. “Potentially combining amyloid- and APOE-targeted therapies to reduce the major plaque pathology in DS is a fascinating implication of this work,” Head told Alzforum.—Chelsea Weidman Burke
References
News Citations
- Gearing Up for Down’s Syndrome Clinical Trials
- Plasma Aβ Test Wins Approval—Are p-Tau Tests Far Behind?
- In Down's Syndrome, Amyloid Vaccine Opens Door to Trials
Therapeutics Citations
Paper Citations
- Fortea J, Vilaplana E, Carmona-Iragui M, Benejam B, Videla L, Barroeta I, Fernández S, Altuna M, Pegueroles J, Montal V, Valldeneu S, Giménez S, González-Ortiz S, Muñoz L, Estellés T, Illán-Gala I, Belbin O, Camacho V, Wilson LR, Annus T, Osorio RS, Videla S, Lehmann S, Holland AJ, Alcolea D, Clarimón J, Zaman SH, Blesa R, Lleó A. Clinical and biomarker changes of Alzheimer's disease in adults with Down syndrome: a cross-sectional study. Lancet. 2020 Jun 27;395(10242):1988-1997. PubMed.
- Prasher VP, Sajith SG, Rees SD, Patel A, Tewari S, Schupf N, Zigman WB. Significant effect of APOE epsilon 4 genotype on the risk of dementia in Alzheimer's disease and mortality in persons with Down syndrome. Int J Geriatr Psychiatry. 2008 Nov;23(11):1134-40. PubMed.
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993 Aug 13;261(5123):921-3. PubMed.
- Royston MC, Mann D, Pickering-Brown S, Owen F, Perry R, Ragbavan R, Khin-Nu C, Tyner S, Day K, Crook R, Hardy J, Roberts GW. ApoE2 allele, Down's syndrome, and dementia. Ann N Y Acad Sci. 1996 Jan 17;777:255-9. PubMed.
External Citations
Further Reading
Papers
- D'Souza H, Mason L, Mok KY, Startin CM, Hamburg S, Hithersay R, Baksh RA, Hardy J, Strydom A, Thomas MS, London Down Syndrome (LonDownS) Consortium. Differential Associations of Apolipoprotein E ε4 Genotype With Attentional Abilities Across the Life Span of Individuals With Down Syndrome. JAMA Netw Open. 2020 Sep 1;3(9):e2018221. PubMed.
Primary Papers
- Bejanin A, Iulita MF, Vilaplana E, Carmona-Iragui M, Benejam B, Videla L, Barroeta I, Fernandez S, Altuna M, Pegueroles J, Montal V, Valldeneu S, Giménez S, González-Ortiz S, Muñoz L, Padilla C, Aranha MR, Estellés T, Illán-Gala I, Belbin O, Camacho V, Wilson LR, Annus T, Osorio RS, Videla S, Lehmann S, Holland AJ, Zetterberg H, Blennow K, Alcolea D, Clarimon J, Zaman SH, Blesa R, Lleó A, Fortea J. Association of Apolipoprotein E ɛ4 Allele With Clinical and Multimodal Biomarker Changes of Alzheimer Disease in Adults With Down Syndrome. JAMA Neurol. 2021 Aug 1;78(8):937-947. PubMed.
- Lemere CA, Head E, Holtzman DM. APOE ε4 Association With Cognition and Alzheimer Disease Biomarkers in Down Syndrome-Implications for Clinical Trials and Treatments for All. JAMA Neurol. 2021 Aug 1;78(8):913-915. PubMed.
- Lleó A, Zetterberg H, Pegueroles J, Karikari TK, Carmona-Iragui M, Ashton NJ, Montal V, Barroeta I, Lantero-Rodríguez J, Videla L, Altuna M, Benejam B, Fernandez S, Valldeneu S, Garzón D, Bejanin A, Iulita MF, Camacho V, Medrano-Martorell S, Belbin O, Clarimon J, Lehmann S, Alcolea D, Blesa R, Blennow K, Fortea J. Phosphorylated tau181 in plasma as a potential biomarker for Alzheimer's disease in adults with Down syndrome. Nat Commun. 2021 Jul 14;12(1):4304. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Southern California Keck School of Mediicine
This important paper from the Fortea and Zaman groups further strengthens the notion that AD in DS shares many similar features to other forms of AD (i.e., sporadic and autosomal-dominant AD), including the increased risk associated with the APOE4 allele.
The authors present data that were collected from a dual center study of adults with DS (n=464). They report that APOE4 carriers presented with AD symptoms at a younger age (50.7 vs. 52.7 years) and showed differences in standard AD biomarker trajectories (Aβ measures in CSF and PET and atrophy on MRI), similar to what is observed in other forms of AD. Interestingly, they did not find differences between APOE4 carriers and noncarriers with regard to NfL fluid biomarkers or CSF total tau or p-tau-181.
The authors specifically report that the APOE4 allele was associated with age-associated changes in all three categories of the ATN framework (amyloid, tau, and neurodegeneration). The associations with amyloid pathology appeared to be the earliest (differences in the early 20s for CSF Aβ42-to-Aβ40 ratio and in the mid-30s for amyloid PET), the greatest, and the most consistent across the different biomarkers. In addition, they found lower levels of CSF Aβ42 and CSF Aβ42-to-Aβ40 ratio associated with the APOE4 allele. These finding reproduce previous observations in both sporadic AD and autosomal-dominant AD.
The relationship between the APOE4 allele and AD risk was first made by Elizabeth Corder, Warren Strittmatter, and the late Allen Roses and their colleagues in a set of landmark studies (Corder et al., 1993; Strittmatter et al., 1993; Saunders et al, 1993). I vividly recall being in the audience at the SFN meeting in Washington, D.C., when the data was first presented. The scientific debate was intense yet inspiring, and contributed to my own interest in entering the field of AD research while an undergraduate student.
The following year, it was demonstrated that the APOE2 allele was protective against AD (Corder et al., 1994). Since then, thousands of papers have been published on the relationship between AD and APOE, though there is still no consensus on a mechanistic explanation for this genetic association. It also remains to be confirmed if the APOE2 allele is also protective against AD in DS, although there has been some work indicating that this may indeed be the case (Royston et al, 1996).
This paper by Bejanin reinforces the idea that stratification based on APOE4 carrier status will be critical to consider when designing clinical trials for AD in DS. In addition, the impact of APOE4 on biological markers of AD offers potential new therapeutic targets for AD in persons with DS.
References:
Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993 Aug 13;261(5123):921-3. PubMed.
Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1977-81. PubMed.
Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC Jr, Rimmler JB, Locke PA, Conneally PM, Schmader KE. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994 Jun;7(2):180-4. PubMed.
Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC Jr, Rimmler JB, Locke PA, Conneally PM, Schmader KE. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994 Jun;7(2):180-4. PubMed.
Royston MC, Mann D, Pickering-Brown S, Owen F, Perry R, Ragbavan R, Khin-Nu C, Tyner S, Day K, Crook R, Hardy J, Roberts GW. ApoE2 allele, Down's syndrome, and dementia. Ann N Y Acad Sci. 1996 Jan 17;777:255-9. PubMed.
King's College London
The findings here are in keeping with previous studies that have shown the effect of APOE E4 alleles in aging adults with Down’s syndrome, with an earlier onset of cognitive decline. The addition of biomarker data in this report confirm it may be associated with differences in early markers of the Alzheimer's disease pathological process.
An intriguing finding from our work is that APOE E4 alleles may confer a cognitive advantage in early life, which is perhaps more pronounced in individuals with DS, who have a background of delayed development, in contrast to the earlier decline in later life (for details see D’Souza et al., 2020).
References:
D'Souza H, Mason L, Mok KY, Startin CM, Hamburg S, Hithersay R, Baksh RA, Hardy J, Strydom A, Thomas MS, London Down Syndrome (LonDownS) Consortium. Differential Associations of Apolipoprotein E ε4 Genotype With Attentional Abilities Across the Life Span of Individuals With Down Syndrome. JAMA Netw Open. 2020 Sep 1;3(9):e2018221. PubMed.
University of Wisconsin-Madison
This excellent, definitive, and timely study, in a large sample of individuals with Down's syndrome, carefully examines if APOe4 effects are consistent with findings in non-DS populations. This work is highly important. It not only confirms the results of previous studies revealing an earlier age on dementia onset associated with e4 carriers—in addition to stratifying prevalence based on age—but also informs us on differences in AD-related biomarkers between carriers and noncarriers. This information on neuroimaging and fluid biomarkers is critical for characterizing the AT(N) framework in DS and for the design and conduct of clinical intervention trials
Make a Comment
To make a comment you must login or register.