Antioxidant Response Might Explain Protective Tau Allele
Quick Links
Tau expression patterns might change in response to oxidative stress, according to an analysis in the April 14 Cell Reports online. Bioinformatics researchers at the National Institute of Environmental Health Sciences (NIEHS) in Research Triangle Park, North Carolina, identified a binding site for the antioxidant response transcription factor NRF2 in the first intron of the tau gene MAPT. About one-quarter of Europeans possess a MAPT genetic variant, the H2 haplotype, that binds NRF2 more tightly. The haplotype’s response to NRF2 signaling might explain why it confers protection against parkinsonism, and potentially other neurodegenerative diseases, suggest senior author Douglas Bell and colleagues.
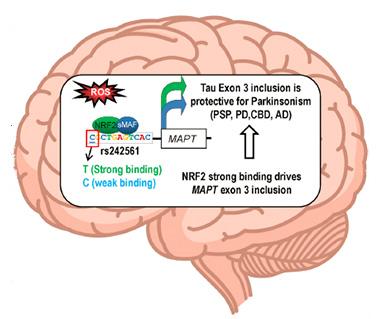
Brain booster.
Authors propose that a variant of the tau gene binds particularly tightly to NRF2 and sMAF during times of oxidative stress, prompting production of less aggregation-prone tau. [Cell Reports, Wang et al.]
The authors arrived at these conclusions by analyzing data from dozens of genome-wide association studies. While GWAS have linked hundreds of single nucleotide polymorphisms (SNPs) to risk for diverse diseases, these SNPS are often not the risk allele, but are merely inherited with the risk alleles themselves because they lie very close together in the genome. Many of the true risk variants lie in regulatory elements of genes, but it has been difficult to correlate such SNPs with disease, said Mark Cookson of the National Institute on Aging in Bethesda, Maryland. Cookson, who was not involved in Bell’s study, praised the authors for finding a way.
First author Xuting Wang and colleagues focused on genes regulated by NRF2. During oxidative stress, NRF2 complexes with other transcription factors called small MAF proteins (sMAFs) to bind genomic sites called antioxidant response elements (AREs). In this way, NRF2/sMAFs activate a slew of genes. Between their prior work and other studies, Bell and colleagues came up with a list of 51,573 NRF2/sMAF binding sites (Chorley et al., 2012). To see if any of these might be linked to diseases, they matched them against 1,016 SNPs from 134 different GWAS on 80 different diseases. They bolstered their SNP list with a further 13,027 variants that are frequently co-inherited with the GWAS SNPs. They found 14 candidate risk SNPs in NRF2 or sMAF binding sites.
The most significant hit on the list lay in the ARE in the first intron of the MAPT gene. The locus contains either a cytosine or a thymine. The thymine variant is part of the H2 haplotype (see May 2004 conference news). GWAS has linked H2 to diminished risk for Parkinson’s, corticobasal syndrome (CBD), progressive supranuclear palsy (PSP), and Alzheimer’s (see Jun 2011 news; Spencer et al., 2011; Kouri et al., 2015; Allen et al., 2014). Could the ARE variant explain the protection afforded by the H2 haplotype?
Knowing the typical ARE consensus sequence, the authors predicted that the thymine version would bind NRF2 more strongly than the cytosine one. To test this, Wang and Bell recruited researchers from the laboratory of Matthew Slattery at the University of Minnesota Medical School in Duluth. They expressed luciferase constructs driven by the tau ARE in a human neuroblastoma cell line and added NRF2. Sure enough, the constructs containing the thymine variant produced twice as much luciferase as the cytosine variant. Wang also checked several human gene expression datasets, and observed that the H2 haplotype was associated with more robust MAPT expression (Grundberg et al., 2012; Ramasamy et al., 2014; GTEx Consortium, 2015; braineac.org).
To test if this regulatory system responds to physiologic changes in vivo, the authors called in Steven Kleeberger and colleagues, also at the NIEHS. The researchers exposed mice to high-oxygen conditions that normally activate NRF2 and drive expression of downstream genes. The hyperoxia elevated tau expression in the cerebellum of wild-type mice by about 70 percent. NRF2 knockouts had no change in tau expression.
About 24 percent of Europeans carry at least one H2 allele. Previous studies found that it not only increases tau levels, it also increases expression of an isoform containing MAPT’s third exon (Caffrey et al., 2008; Trabzuni et al., 2012). Tau containing exon 3 protects mice against neurodegeneration because it is less likely to aggregate than other isoforms, and there are hints it protects against Aβ toxicity in mouse models of Alzheimer’s (Zhong et al., 2012; Ittner et al., 2011). Wang and colleagues hypothesize that the NRF2 binding site explains the protective effects of the H2 haplotype, because it amplifies expression of the less aggregation-prone tau.
“It is a reasonable hypothesis,” said Cookson. He cautioned that many other variants are co-inherited with the H2 haplotype, so any of those might also contribute to its protective effects. The proposed link between NRF2, the H2 haplotype, and resistance to tauopathy may be difficult to prove, he said. Cookson suggested that researchers could use iPS-derived neurons to test the effects of different variants.
Wang has a different idea in mind to test his theory. He noted that certain foods, such as broccoli and cauliflower, contain NRF2 activators. He predicts that people, particularly those with the H2 haplotype, who regularly munch those cruciferous vegetables would be less likely to develop PD, PSP, or CBD. He and Bell would like to collaborate with epidemiologists to test this hypothesis, he said. He also hopes to correlate oxidative stress and protection from tauopathy in mouse models. Even for people without the H2 haplotype, future therapeutic activators of NRF2 might be beneficial, Wang said, since NRF2 also binds the cytosine variant.
Why it would be beneficial to activate tau in response to oxidative stress? Wang is not sure. He noted that NRF2 is generally protective, and speculated that if NRF2, in the protective haplotype, keeps tau levels high, this would stabilize microtubules in times of cell stress. Being better able to activate different tau isoforms might give neurons nuanced control over their cytoskeletons, Cookson theorized.
“More studies need to be done to verify these observations, but they are a great start,” commented Jeffrey and Delinda Johnson of the University of Wisconsin-Madison. “The development of therapeutics targeting this pathway is critical,” they claim. Already, scientists have found that NRF2 activation mitigates some of the pathologies associated with PD, AD, Huntington’s, and amyotrophic lateral sclerosis (reviewed in Johnson and Johnson, 2015).—Amber Dance
References
News Citations
- St. Moritz: Part 3. This Research Isn't Folding Up: Genetics, Transport, Seeding, Protein Microscopy
- GWAS Fingers Tau and Other Genes for Parkinsonian Tauopathy
Paper Citations
- Chorley BN, Campbell MR, Wang X, Karaca M, Sambandan D, Bangura F, Xue P, Pi J, Kleeberger SR, Bell DA. Identification of novel NRF2-regulated genes by ChIP-Seq: influence on retinoid X receptor alpha. Nucleic Acids Res. 2012 Aug;40(15):7416-29. Epub 2012 May 11 PubMed.
- UK Parkinson's Disease Consortium, Wellcome Trust Case Control Consortium 2, Spencer CC, Plagnol V, Strange A, Gardner M, Paisan-Ruiz C, Band G, Barker RA, Bellenguez C, Bhatia K, Blackburn H, Blackwell JM, Bramon E, Brown MA, Brown MA, Burn D, Casas JP, Chinnery PF, Clarke CE, Corvin A, Craddock N, Deloukas P, Edkins S, Evans J, Freeman C, Gray E, Hardy J, Hudson G, Hunt S, Jankowski J, Langford C, Lees AJ, Markus HS, Mathew CG, McCarthy MI, Morrison KE, Palmer CN, Pearson JP, Peltonen L, Pirinen M, Plomin R, Potter S, Rautanen A, Sawcer SJ, Su Z, Trembath RC, Viswanathan AC, Williams NW, Morris HR, Donnelly P, Wood NW. Dissection of the genetics of Parkinson's disease identifies an additional association 5' of SNCA and multiple associated haplotypes at 17q21. Hum Mol Genet. 2011 Jan 15;20(2):345-53. Epub 2010 Nov 2 PubMed.
- Kouri N, Ross OA, Dombroski B, Younkin CS, Serie DJ, Soto-Ortolaza A, Baker M, Finch NC, Yoon H, Kim J, Fujioka S, McLean CA, Ghetti B, Spina S, Cantwell LB, Farlow MR, Grafman J, Huey ED, Ryung Han M, Beecher S, Geller ET, Kretzschmar HA, Roeber S, Gearing M, Juncos JL, Vonsattel JP, Van Deerlin VM, Grossman M, Hurtig HI, Gross RG, Arnold SE, Trojanowski JQ, Lee VM, Wenning GK, White CL, Höglinger GU, Müller U, Devlin B, Golbe LI, Crook J, Parisi JE, Boeve BF, Josephs KA, Wszolek ZK, Uitti RJ, Graff-Radford NR, Litvan I, Younkin SG, Wang LS, Ertekin-Taner N, Rademakers R, Hakonarsen H, Schellenberg GD, Dickson DW. Genome-wide association study of corticobasal degeneration identifies risk variants shared with progressive supranuclear palsy. Nat Commun. 2015 Jun 16;6:7247. PubMed.
- Allen M, Kachadoorian M, Quicksall Z, Zou F, Chai HS, Younkin C, Crook JE, Pankratz VS, Carrasquillo MM, Krishnan S, Nguyen T, Ma L, Malphrus K, Lincoln S, Bisceglio G, Kolbert CP, Jen J, Mukherjee S, Kauwe JK, Crane PK, Haines JL, Mayeux R, Pericak-Vance MA, Farrer LA, Schellenberg GD, Parisi JE, Petersen RC, Graff-Radford NR, Dickson DW, Younkin SG, Ertekin-Taner N. Association of MAPT haplotypes with Alzheimer's disease risk and MAPT brain gene expression levels. Alzheimers Res Ther. 2014;6(4):39. Epub 2014 Jul 1 PubMed.
- Grundberg E, Small KS, Hedman ÅK, Nica AC, Buil A, Keildson S, Bell JT, Yang TP, Meduri E, Barrett A, Nisbett J, Sekowska M, Wilk A, Shin SY, Glass D, Travers M, Min JL, Ring S, Ho K, Thorleifsson G, Kong A, Thorsteindottir U, Ainali C, Dimas AS, Hassanali N, Ingle C, Knowles D, Krestyaninova M, Lowe CE, Di Meglio P, Montgomery SB, Parts L, Potter S, Surdulescu G, Tsaprouni L, Tsoka S, Bataille V, Durbin R, Nestle FO, O'Rahilly S, Soranzo N, Lindgren CM, Zondervan KT, Ahmadi KR, Schadt EE, Stefansson K, Smith GD, McCarthy MI, Deloukas P, Dermitzakis ET, Spector TD, Multiple Tissue Human Expression Resource (MuTHER) Consortium. Mapping cis- and trans-regulatory effects across multiple tissues in twins. Nat Genet. 2012 Oct;44(10):1084-9. Epub 2012 Sep 2 PubMed.
- Ramasamy A, Trabzuni D, Guelfi S, Varghese V, Smith C, Walker R, De T, UK Brain Expression Consortium, North American Brain Expression Consortium, Coin L, de Silva R, Cookson MR, Singleton AB, Hardy J, Ryten M, Weale ME, UK Brain Expression Consortium, North American Brain Expression Consortium. Genetic variability in the regulation of gene expression in ten regions of the human brain. Nat Neurosci. 2014 Oct;17(10):1418-28. Epub 2014 Aug 31 PubMed.
- GTEx Consortium. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015 May 8;348(6235):648-60. Epub 2015 May 7 PubMed.
- Caffrey TM, Joachim C, Wade-Martins R. Haplotype-specific expression of the N-terminal exons 2 and 3 at the human MAPT locus. Neurobiol Aging. 2008 Dec;29(12):1923-9. PubMed.
- Trabzuni D, Wray S, Vandrovcova J, Ramasamy A, Walker R, Smith C, Luk C, Gibbs JR, Dillman A, Hernandez DG, Arepalli S, Singleton AB, Cookson MR, Pittman AM, de Silva R, Weale ME, Hardy J, Ryten M. MAPT expression and splicing is differentially regulated by brain region: relation to genotype and implication for tauopathies. Hum Mol Genet. 2012 Sep 15;21(18):4094-103. Epub 2012 Jun 20 PubMed.
- Zhong Q, Congdon EE, Nagaraja HN, Kuret J. Tau isoform composition influences rate and extent of filament formation. J Biol Chem. 2012 Jun 8;287(24):20711-9. PubMed.
- Ittner A, Ke YD, Eersel J, Gladbach A, Götz J, Ittner LM. Brief update on different roles of tau in neurodegeneration. IUBMB Life. 2011 Jul;63(7):495-502. PubMed.
- Johnson DA, Johnson JA. Nrf2--a therapeutic target for the treatment of neurodegenerative diseases. Free Radic Biol Med. 2015 Nov;88(Pt B):253-67. Epub 2015 Aug 14 PubMed.
External Citations
Further Reading
Papers
- Carvalho AN, Firuzi O, Gama MJ, van Horssen J, Saso L. Oxidative stress and antioxidants in neurological diseases: is there still hope?. Curr Drug Targets. 2016 Apr 1; PubMed.
- Esteras N, Dinkova-Kostova AT, Abramov AY. Nrf2 activation in the treatment of neurodegenerative diseases: a focus on its role in mitochondrial bioenergetics and function. Biol Chem. 2016 May 1;397(5):383-400. PubMed.
- Denzer I, Münch G, Friedland K. Modulation of mitochondrial dysfunction in neurodegenerative diseases via activation of nuclear factor erythroid-2-related factor 2 by food-derived compounds. Pharmacol Res. 2016 Jan;103:80-94. Epub 2015 Nov 25 PubMed.
- Lee JA, Son HJ, Park KD, Han SH, Shin N, Kim JH, Kim HR, Kim DJ, Hwang O. A Novel Compound ITC-3 Activates the Nrf2 Signaling and Provides Neuroprotection in Parkinson's Disease Models. Neurotox Res. 2015 Nov;28(4):332-45. Epub 2015 Aug 2 PubMed.
- Yamazaki H, Tanji K, Wakabayashi K, Matsuura S, Itoh K. Role of the Keap1/Nrf2 pathway in neurodegenerative diseases. Pathol Int. 2015 May;65(5):210-9. Epub 2015 Feb 23 PubMed.
- Yang Y, Jiang S, Yan J, Li Y, Xin Z, Lin Y, Qu Y. An overview of the molecular mechanisms and novel roles of Nrf2 in neurodegenerative disorders. Cytokine Growth Factor Rev. 2015 Feb;26(1):47-57. Epub 2014 Sep 16 PubMed.
News
- Tau Gene Confirmed to Amp Up Alzheimer’s Risk and Neurodegeneration
- Et tu, Brute? Parkinson’s GWAS Fingers Tau Next to α-Synuclein
- New Genetics Frontiers: Finding Modifiers, Making Sense of Pathways
- Genentech Strikes Deal with 23andMe to Study Parkinson’s Genomes
- Researchers Build on GWAS to Parse Genetic Players in AD and PD
Primary Papers
- Wang X, Campbell MR, Lacher SE, Cho HY, Wan M, Crowl CL, Chorley BN, Bond GL, Kleeberger SR, Slattery M, Bell DA. A Polymorphic Antioxidant Response Element Links NRF2/sMAF Binding to Enhanced MAPT Expression and Reduced Risk of Parkinsonian Disorders. Cell Rep. 2016 Apr 13; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.