Lecanemab Post Hoc: Is Continual Treatment Required for Cognitive Benefit?
Quick Links
Once again, Aβ immunotherapy was the topic du jour at this year’s Alzheimer’s Association International Conference (AAIC), held July 26-30 in Denver and online. Drug companies are moving to Phase 3 studies while simultaneously wooing the FDA for accelerated approval. Among their proffers is lecanemab, an antibody trained against protofibrillar forms of Aβ. After a complicated Bayesian Phase 2 study hinted at efficacy in people with early AD, and participants had been off lecanemab for nine months to five years, Eisai began an open-label extension (OLE).
- Lecanemab treatment slowed cognitive decline during a Phase 2 study.
- When dosing was paused, cognitive slide accelerated, but the treatment group still did better than controls.
- In a small open-label extension, decline appeared to slow again.
- Plasma Aβ42/40 ratio rose on treatment, fell off treatment.
At AAIC, Chad Swanson, who directs the company’s neuroscience clinical development group in Woodcliff Lake, New Jersey, reported 18-month biomarker and cognitive data from this small OLE, and post hoc analyses across the entire span of the study. He reported that cognition declined more slowly in people on lecanemab than in controls. While decline regained speed during time off drug, people who had received treatment still maintained their edge over those who had been in the placebo group. Open-label treatment slowed cognitive decline once again. The plasma ratio of Aβ42/40 gradually fell during the gap between the blinded and open-label trials—indicating a rise in brain amyloid deposition—but rebounded when open-label treatment resumed. Together, the findings hint at a disease-modifying effect, claimed Swanson. Whether continued dosing would be necessary to maintain a cognitive benefit remains unsettled.
Lecanemab’s Rocky Road
This antibody's path through development has been bumpy. Eisai employed an ambitious Bayesian design for its 856-participant Phase 2 trial. In this type of trial, data are continually measured and enrollees are increasingly randomized to the more-effective doses as the trial progresses. The 18-month trial started with five dose groups and a placebo group. Although the trial missed its 12-month cognitive endpoint, data at 18 months indicated that the highest dose—10 mg/kg bi-weekly—had lowered amyloid burden by 93 percent and slowed, but did not halt, cognitive decline (Jul 2018 conference news).
This dose slowed worsening on the ADCOMS by 30 percent, and nearly halved decline on the ADAS-Cog. That’s similar to donanemab, which docked decline on the iADRS, a cognitive and functional composite measure, by 32 percent in Phase 2. Aducanumab slowed decline on the ADAS-Cog by a quarter in the Phase 3 EMERGE, but had no benefit in ENGAGE (Aug 2021 conference news; Dec 2019 conference news).
The trouble for lecanemab: an uneven distribution of ApoE4 carriers among the dose groups muddled interpretation of the data for a time (Nov 2018 conference news). This kink sorted out, Eisai started the OLE, inviting those who had completed the placebo-controlled, double-blinded “core” portion of the trial to now take the highest, 10 mg/kg twice-monthly dose for another five years. Researchers previously reported that during the gap period between the placebo-controlled and extension—which ranged from nine to 59 months—cognition declined at a similar rate among all groups, although those who had received either of the two highest doses of lecanemab maintained their edge over the placebo group (Dec 2019 conference news). Their amyloid burden also remained low. The data indicated that while plaques had remained at bay during the gap period, cognition had started to wane again, suggesting that continued treatment, perhaps to remove Aβ protofibrils, may be necessary for an enduring cognitive effect.
Further, at last year’s CTAD, Swanson reported amyloid-PET data as far as 12 months into the OLE (Nov 2020 conference news). Among participants who had been on placebo in the trial's core portion and were therefore getting lecanemab for the first time, amyloid dropped to a similar degree as it had among participants treated in the core trial.
At AAIC, then, Swanson reported 18-month cognitive and biomarker data from the OLE. In toto, 180 participants enrolled. At baseline, 24 scored greater than 1 on the clinical dementia rating (CDR) scale, meaning that they had progressed beyond the realm of early AD. The core study had enrolled people with MCI or mild AD (CDR 0.5-1), meaning that these 24 no longer met inclusion criteria for the core trial. Despite this, these progressors, now with moderate to severe AD, continued to receive treatment in the extension.
Swanson presented retrospective data on them. Showing pooled data from people who had been in the placebo and treatment groups, he reported that these 24 progressors had also declined at a more rapid pace on other measures of cognition, namely the ADCOMS, CDR-SB, and ADAS-Cog, during the core and gap periods, compared to the 147 participants who were still at the early AD stage by the beginning of the OLE. Swanson did not break down the 24 progressors by treatment group in his presentation, but he told Alzforum that lecanemab treatment did not influence their rate of cognitive decline, either in the core or later in the extension. Essentially, these participants did not respond to lecanemab treatment. Swanson said that efforts are underway to investigate whether baseline biomarkers, including tau PET or fluid biomarkers, could have identified this group of non-responders from the beginning of the trial.

Non-Responders Identified. Of 180 people in the open-label extension, the 24 whose global CDR was 1 or higher at baseline (red line) had declined more rapidly on ADCOMS during the core (blue) and gap (orange) periods of the trial than the people who started the OLE with milder dementia (blue line). Pink section reflects a three-month safety follow-up. [Image courtesy of Biogen/Eisai.]
Swanson excluded the 24 fast progressors from subsequent analyses. Among the remaining participants, those who had been in the placebo group from the get-go had worsened during the core trial on ADCOMS at a similar rate as did people with mild AD in the ADNI cohort. On the other hand, people in the two treatment groups had worsened at a slower rate that those historical controls. Moving on to the gap period, Swanson showed that decline continued apace among all three groups, with the placebo group continuing to do worse, and the lecanemab groups doing better than ADNI controls. This suggests an enduring disease-modifying effect, Swanson said. These progression rates in the gap period were based on measures taken at a single time point at the beginning of the OLE; the gap period varied widely.
What happened during the extension itself? Swanson presented data from a subset of 62 people who had completed 18-month cognitive assessments in the OLE, and had been in the placebo, 10 mg/kg monthly, or 10 mg/kg biweekly groups during the core. This group excluded the fast progressors. Measuring change in ADCOMS relative to OLE baseline, Swanson found that while the people who had previously been on placebo and switched to high-dose lecanemab initially declined like ADNI controls, lo and behold, this worsening slowed after six months and plateaued through 18 months, suggesting the immunotherapy was having an effect (see image below).

Stable in Open Label? In the OLE, everyone took the highest dose of lecanemab. People who had been on placebo (black) or the highest dose (green) in the core study, declined more slowly on ADCOMS after six months, while those who had been on the second-highest dose in the core (blue) continued to decline, though not as fast as ADNI controls (orange). People in the second-highest dose group in the core trial were more likely to be ApoE4 carriers. [Image courtesy of Biogen/Eisai.]
The drug similarly altered the rate of cognitive decline in people who had previously taken the highest dose of the drug, showing stabilization after six months. However, those in the second-highest dose group behaved more like ADNI controls, continuing to decline over the 18 months. Notably, this group had a higher proportion of ApoE4 carriers than the other two groups.
Swanson showed no absolute scores, only change from OLE baseline. The data were also based on small numbers of participants, and Swanson emphasized that the primary purpose of the analysis was to generate hypotheses that could be tested in the ongoing Phase 3 study, CLARITY-AD.
How did lecanemab influence Aβ accumulation throughout the trial? Swanson had shown previously that the highest dose of lecanemab had lowered plaque burden by more than 90 percent, as per PET, and that amyloid deposits had largely remained at bay during the gap period. At AAIC, he offered more support for that finding, reporting ratios of Aβ42 to Aβ40 in the plasma, which had been collected in both core and extension periods. As more Aβ42 gets released from fibrils and plaque decorated with antibodies, scientists expect the plasma level to rise. In keeping with this, plasma Aβ42/40 held steady in the placebo group during the trial's core period, but rose in the two high-dose treatment groups. Between the end of the core period and the beginning of the OLE, the ratio had crept downward in the treatment groups, suggesting a surge of amyloid deposition off drug. Even so, the ratio remained well above that in placebo (see image below).
Six months into the extension, the Aβ42/40 ratio of the people who had switched from placebo to high dose had nearly caught up with those of the two previously treated groups, suggesting a rapid cessation of amyloid deposition.
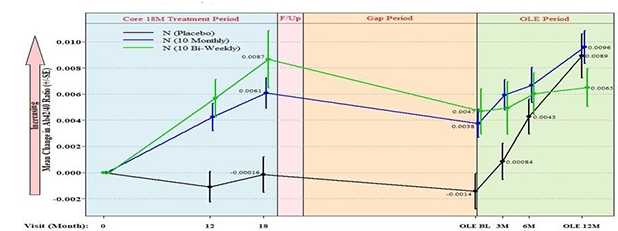
Shifty Plasma. During the trial's core period (blue), the ratio of Aβ42/Aβ40 increased in the two treatment groups (green, blue lines) but held steady in the placebo group (black line). The ratio fell between the core and OLE (yellow), then rose again once treatment resumed. [Courtesy of Eisai.]
“While the clinical treatment effects in the OLE are difficult to evaluate with very small numbers and absent a control group, the movement of the plasma Aβ42/40 ratio off and on treatment is potentially of considerable importance and merits significantly further investigation and replication,” wrote Howard Feldman of the University of California, San Diego, to Alzforum.
To Swanson, the Aβ42/40 data suggest lecanemab effectively stops Aβ accumulation. However, once treatment ends, Aβ starts accumulating again, even if plaques are not yet detectable. He interpreted both the biomarker and cognitive findings as evidence that continued treatment with lecanemab may be necessary to keep Aβ build-up, and cognitive deterioration, at bay.
David Morgan of the Michigan State University in Grand Rapids interpreted the data differently. He noted that while plasma Aβ42/40 did dip during the gap period, it remained well above the placebo group. He made a similar point about the cognitive data, noting that the differences between placebo and treatment groups were maintained throughout the gap period. To his mind, this implies a continued disease-modifying effect in the absence of treatment. By comparison, in a Phase 2 trial of donanemab, plaques dropped to the level of healthy controls, and were still low a year after treatment was stopped (see Part 2 of this series). Cognitive benefit still persisted after treatment had stopped, as well.
Henrik Zetterberg of the University of Gothenberg in Sweden hesitated interpreting the plasma Aβ42/40 findings. He noted that in previous trials of Aβ monoclonals, the antibodies stabilized Aβ in the plasma, thus increasing the peptide's half-life there. Without data on the absolute concentrations of Aβ42 and Aβ40, it is unclear whether the plasma ratio truly reflects changes in Aβ accumulation in the brain, he cautioned.
In addition to the ongoing extension study, two Phase 3 studies are putting lecanemab to the test. In March 2019, CLARITY-AD started enrolling people with mild AD into an 18-month early AD trial, whose primary outcome is change on the CDR-SB. At AAIC, Eisai's Michelle Gee showed the baseline characteristics of participants enrolled thus far. Of more than 1,700 projected participants, 1,536 have been selected at sites in North America, Europe, Asia, and Australia. For the most part, the randomized population seems very like the participants in the Phase 2 study, with an average age of 72 and cognitive scores indicative of early AD. Enrollment is complete in all countries bar China, where it continues. Preliminary results are expected in September 2022.
The more recent AHEAD 3-45 secondary prevention study is seeking cognitively normal people with signs of amyloid in the brain (Nov 2020 conference news). AHEAD 3-45 combines two studies, A3 and A45. A3 is evaluating lecanemab's ability to lower plaque in people with only 20 to 40 centiloids of amyloid, and has a primary endpoint of amyloid reduction. The Phase 3 A45 enrolls people with intermediate levels of amyloid—above 40 centiloids—and uses a cognitive endpoint, the PACC-5.
At AAIC, Jin Zhou of Eisai reported that this four-year trial, run by Eisai and the Alzheimer’s Clinical Trial Consortium (ACTC) has so far screened 881 people. Of those, 209 were excluded prior to receiving an amyloid-PET scan, based on general health or cognition. Of the remaining 672 who received an amyloid-PET scan, 297 were excluded because their amyloid levels were too low, leaving only one third eligible for the trial. Amyloid-PET was by far the most common reason for exclusion. While a computational simulation based on ADNI data had projected that about half of screened participants would meet the amyloid-PET selection criteria, in reality only 32 percent have, so far. As of June 30, 2021, 77 people have been randomized—23 people had enrolled in A3, and 54 in A45, Zhou reported.
Even as the Phase 3 trials push ahead, Biogen/Eisai recently announced plans to apply for accelerated approval of lecanemab (Endpoint News), following in Eli Lilly’s footsteps with donanemab.—Jessica Shugart
References
Therapeutics Citations
News Citations
- BAN2401 Removes Brain Amyloid, Possibly Slows Cognitive Decline
- On Donanemab, Plaques Plummet. Off Donanemab, They Stay Away
- Exposure, Exposure, Exposure? At CTAD, Aducanumab Scientists Make a Case
- Second Look at BAN2401 Data Still Positive, Despite Snafu
- Amyloid Clearance: Check. Cognitive Benefit: Um … Maybe.
- BANish Aβ? BAN2401 Antibody Makes Its Move in Phase 3 Program
- TRC-PAD Funnel Finally Touches Down
External Citations
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.