CTAD Lessons for 2020: More Phase 2 Trials, More Diversity
Quick Links
What lies ahead for Alzheimer’s therapy development? While anti-amyloid antibodies are at last signaling some success, researchers agree that these expensive—and, thus far, at best modestly effective—biologic drugs can form only part of the arsenal needed to fight the disease. Researchers at the 12th Clinical Trials on Alzheimer’s Disease conference, held December 4–7 in San Diego, California, broadly agreed that an array of therapeutic approaches will be needed to target symptomatic stages, or to combine with antibodies to boost efficacy. Speakers also discussed how to improve the dismal success rate of Alzheimer’s clinical trials. In particular, there is a push to spend more time in Phase 2 to find the right dose and confirm physiological effects of the drug at hand. Others argued that machine-learning approaches, which have made a slow and steady entrance into Alzheimerology over the past five years or so, are now robust enough to make trials shorter, faster, and more efficient.
- Funding agencies encourage exploration of more diverse therapeutic targets.
- A return to getting efficacy data in Phase 2 may lower failure rate in Phase 3.
- Machine learning could help slim down and streamline trials.
The mood overall was upbeat. The crowd in San Diego seemed confident that the field now has the tools it needs to move forward, given recent investments in infrastructure and basic research into the systems biology of AD. That research suggests that therapeutic approaches should target not only Aβ and tau pathologies, but inflammation, vascular dysfunction, and multiple proteinopathies, as well. In addition, research into resilience factors might point toward ways to strengthen function in the aging brain. Eliezer Masliah, who heads the neuroscience division at the National Institute on Aging, struck a particularly bullish note in his keynote address. “We have a blueprint for how to get to a cure for AD by 2025. I feel very confident we’ll make that goal.”
Increased funding for Alzheimer’s research powers his plan. Masliah noted that in 2019 the allocation for AD and related diseases was $2.3 billion, more than four times the 2013 total. Although this still lags behind the $6 billion spent on cancer research, the additional funding has allowed the NIA to invest in major infrastructure initiatives, such as the MODEL-AD program for generating better animal models, and the Alzheimer’s Disease Clinical Trials Consortium to support clinical programs (Jan 2017 news; Dec 2017 news).
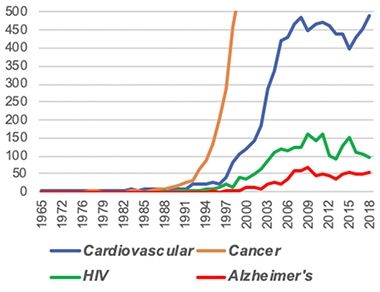
Far To Go. The roughly 50 Alzheimer’s trials each year represent a drop in the bucket compared with the 500 for cardiovascular disease and 2,500 for cancer. [Courtesy of NIA.]
The challenge is to convert these resources into actual trials. There are only 50 to 60 AD trials each year, compared with 500 for cardiovascular disease and 2,500 for cancer. Masliah stressed that the AD field needs more shots on goal. NIA believes the key is to diversify and fill up the therapeutic pipeline with a greater variety of targets.
The agency currently funds a dozen different target areas, including tau, ApoE, neuroprotection, and neurotransmitter receptors. Its priorities have changed over time. For example, since 2014, agency funding for amyloid-directed therapies has fallen from 25 percent of the portfolio to less than 10 percent, while funding for inflammation has risen sharply, from about 5 to 20 percent of the portfolio. Is the recent announcement of positive Phase 3 results for aducanumab going to change the agency’s direction? Joe Balintfy, a spokesperson at NIA, said this is unlikely, noting that the agency’ priorities are set in consultation with research leaders at the annual NIH summits mandated by the National Alzheimer’s Project Act (Mar 2018 news; Mar 2019).
The Accelerating Medicines Partnership (AMP-AD) is tasked with discovering additional targets by analyzing systems biology in healthy and diseased brain in search of new pathways relevant to AD (Feb 2014 news). The AMP-AD’s Agora database now includes more than 500 candidate targets, ranked by druggability, Masliah said. Of these, 113 are predicted to be druggable with small molecules. These candidate targets typically are components of regulatory networks within the brain. Researchers can view supporting information on each target, such as how its expression changes in AD and whether that correlates with pathology.
To find further disease targets beyond what AMP turns up, NIA also funds projects to investigate the vascular contribution to AD (M2OVE-AD), neuropsychiatric symptoms (NPS-AD), and resilience mechanisms (Resilience-AD).
To move these candidates toward trials, in October 2019 NIA committed $73 million to launch its newest initiative, the AD Translational Centers for New Medicines. This project brings together researchers at diverse institutions into two multidisciplinary teams charged with developing tools such as antibodies and chemical probes directed against candidate targets, and with performing initial investigations in mouse models (Oct 2019 news). “Having a diversified pipeline will put us on the road to 2025,” Masliah concluded.

Changing Priorities. Since 2014, NIA funding for amyloid-related therapies (blue) has shrunk, while that for inflammatory targets (orange) has ballooned. [Courtesy of NIA.]
Phase 2: Reconnecting With a Long-Lost Friend?
Others dryly noted that more trials may not help much if the success rate doesn’t improve. Currently, most Alzheimer’s drug trials are negative. Alette Wessels of Eli Lilly sees a more deliberate focus on Phase 2 as a solution. “We need to do true dose-exploratory Phase 2 studies,” Wessels said in San Diego. Many scientists at CTAD agreed, noting privately that dose-response data and a promising but unclear efficacy signal is what Biogen got out of its Phase 3 program, which followed from Phase 1 trials. Other programs followed a similar strategy, including Lilly’s solanezumab and lanabecestat, Merck’s verubecestat, and Roche’s gantenerumab. A notable exception is BAN2401, which conducted an 856-person Phase 2 study before starting Phase 3.
At CTAD, Wessels stressed that before being advanced into Phase 3, a drug should have formally demonstrated sufficient concentration in the central nervous system, target engagement, a dose response, and downstream physiological effects on the brain, as well as early indications of clinical effects. She suggested Phase 2 trials incorporate indirect measures of cognition, such as EEG or fMRI scans.
Going forward, Phase 2 trials should include adaptive features, Wessels said. This will allow researchers to hone their approach. If Phase 2 results indicate a partial response to drug, such as biomarker movement without clinical benefit, sponsors should stay in Phase 2, adjusting parameters until they see a strong effect, Wessels said. She emphasized the importance of a large effect size in Phase 2, because there tends to be a regression to the mean in the heterogeneous international populations of Phase 3 trials. “Before we initiate Phase 3, we should have an understanding of efficacy,” Wessels said.
Machines to the Rescue?
If this all sounds like a lot of effort and money spent in Phase 2, could machine learning make trials at this stage more efficient? Nicolai Franzmeier of Ludwig Maximilians University, Germany, believes so. He used artificial intelligence to analyze multiple biomarkers, including structural MRI, FDG PET, amyloid PET, and cerebrospinal fluid Aβ/t-tau/p-tau, from a DIAN cohort of 121 cognitively healthy mutation carriers and 54 noncarriers. The goal was to identify the parameter combination that best predicted a participant’s estimated age of symptom onset, aka EYO. Using data from these markers, Franzmeier developed a computer algorithm that fit the bill, predicting EYO with an R2 value of 0.53.
The researchers tested this model in an ADNI cohort comprising 49 amyloid-negative controls and 216 amyloid-positive people with MCI, who were followed for four years. Model predictions explained about 25 percent of the observed decline on the ADAS-Cog13 and ADNI-MEM, i.e., an R2 of about 0.25.
This algorithm could help trialists select people at high risk of decline, Franzmeier said. He estimated that it could lower the number of participants needed to see a treatment benefit by 60 percent; for example, group sizes needed to detect a 20 percent slowing of cognitive decline would drop from 1,982 to 773. An audience member pointed out that collecting so much biomarker data is expensive in itself; Franzmeier said that even using just two of the four markers could cut enrollment in half.
Aaron Smith of Unlearn.AI, a startup company that uses machine learning to facilitate trials, suggested another way digital technology could cut enrollment. Machines can use data from the control arms of past trials to develop predictions for how disease will progress in a person depending on his or her baseline characteristics, he said. In effect, computers can generate a “digital twin” for each enrollee, describing what would likely happen to them without treatment. These digital twins can supplement the physical placebo group of a trial. This would lower the number of participants needed for a given trial, and allow a greater proportion of participants to receive drug rather than placebo. The chance of ending up in the placebo group deters many a potential participant.
Smith and colleagues used 18 months of data on 1,909 patients from the Coalition Against Major Diseases (CAMD) AD database to develop an algorithm for generating digital twins. This “synthetic data” accurately predicted changes in ADAS-Cog scores, and was indistinguishable from actual data by logistic regression analysis, he reported (Fisher et al., 2019). The researchers have since improved this model, now incorporating data from 5,000 people participating in a variety of clinical trial designs, Smith noted.
Would the Food and Drug Administration accept trial results that use digital twins? Smith said his company is discussing their methodology with the FDA, but at this point, he recommends using the approach for Phase 2.
Overall, machine learning is gaining a toehold in the Alzheimer’s field, with 10 talks and 10 posters on the topic at CTAD. Newman Knowlton of Pentara Corporation said in San Diego that artificial intelligence is particularly adept at pattern recognition, making it ideal for applications such as analyzing speech—or indeed classifying patients by disease stage. Computer models can also adjust individual dosing based on pharmacokinetic data, flag potential medication interactions, and predict disease progression. All these applications require machines to synthesize existing data, which is their forte. However, there are some things artificial intelligence cannot do, such as determine drug efficacy, Knowlton noted. For that, human judgment is required.
Considering the future for the Alzheimer’s field, Stephen Salloway of Brown University in Providence, Rhode Island, sounded hopeful. “Working in Alzheimer’s research, you have to keep at it until you break through to the next level,” Salloway told the CTAD audience. He sees the field as being on the cusp of that next level, noting that research networks, such as AIBL, ADNI, DIAN, BioFinder, EPAD, and others, have delivered major progress in brain imaging, biomarker development, and an understanding of the fundamental biology of AD.
To find effective treatments, Salloway believes, the field will need to move beyond paying lip service to combination therapies and start putting those trials in practice. While employing Aβ-targeted therapies earlier in the course of disease, the field must also doggedly scour other biological pathways, such as inflammation and cellular aging, for targets that will form the basis of future combination therapies. Recruiting young scientists from diverse fields into the Alzheimer’s fold will be key to expanding the range of potential treatments, he said. Finally, Salloway stressed that, to grow cohorts for platform-style trials, public engagement in AD trials must increase exponentially. “It needs to go to a much grander scale, where the public really partners with us. Believe me—they are willing. We just need to figure out how to make that partnership flourish,” Salloway said.
Salloway noted the importance of non-pharmacological approaches. Observational studies suggest that living a healthy lifestyle could shave a third off the population dementia risk, and multimodal intervention trials suggest cognitive benefits (see Aug 2019 news; FINGER clinical trial; Nov 2015 news). The ongoing POINTER trial in the U.S. will compare the interventions in different populations. “We’re sure it’s going to be beneficial—we just need to figure out what the regimen is, how to attract people, and how to maintain adherence,” Salloway said.
Laura Baker of Wake Forest University in North Carolina said that POINTER, and similar trials around the globe, will zero in on the most effective interventions suited for each culture. At the same time, better harmonization between trials in terms of which interventions are used and how their cognitive effects are measured is necessary to enable accurate comparisons between lifestyle and pharmacological trials. Baker believes a healthy lifestyle may prepare the body and brain to better respond to drug therapies, asking, “Can lifestyle interventions be a fertilizer for pharmacological therapies?”—Madolyn Bowman Rogers and Jessica Shugart
References
News Citations
- Building Better Mouse Models for Late-Onset Alzheimer’s
- Clinical Trials Consortium Succeeds ADCS, Focuses on Prevention
- Big Data Was the Big Theme at Shortened NIH Summit
- NIH Summit Sets Agenda for AD-Related Dementias
- New Initiative AMPs Up Alzheimer’s Research
- NIH Funds Translational Research Centers to Accelerate AD Drug Discovery
- Healthy Lifestyle Hedges Dementia Risk, but Not if Genetic Risk Runs High
- Health Interventions Boost Cognition—But Do They Delay Dementia?
Paper Citations
- Fisher CK, Smith AM, Walsh JR, Coalition Against Major Diseases, Abbott, Alliance for Aging Research, Alzheimer’s Association, Alzheimer’s Foundation of America, AstraZeneca Pharmaceuticals LP, Bristol-Myers Squibb Company, Critical Path Institute, CHDI Foundation, Inc., Eli Lilly and Company, F. Hoffmann-La Roche Ltd, Forest Research Institute, Genentech, Inc., GlaxoSmithKline, Johnson & Johnson, National Health Council, Novartis Pharmaceuticals Corporation, Parkinson’s Action Network, Parkinson’s Disease Foundation, Pfizer, Inc., sanofi-aventis. Collaborating Organizations: Clinical Data Interchange Standards Consortium (CDISC), Ephibian, Metrum Institute.. Machine learning for comprehensive forecasting of Alzheimer's Disease progression. Sci Rep. 2019 Sep 20;9(1):13622. PubMed.
External Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.