Keystone Meeting on Microglia/Neurodegeneration: Here’s the Buzz
Quick Links
Sometimes arriving even 15 minutes early to a lecture hall can turn out to be, well, too late. Interest was so keen in “Common Mechanisms of Neurodegeneration” and “Microglia in the Brain,” joint Keystone Symposia held June 12-16 in Keystone, Colorado, that attendees were claiming seats well before sessions started with their bags, books, computers, and other personal items. “Tardy” folks scrambled for whatever spots were left at the back of packed hallways, or sat on the floor to be close to the screen. Yet no one complained. A spirit of cooperation and collaboration was palpable at this meeting, as researchers seemed united in a common purpose to figure out why neurons degenerate and what role microglia play in that process and indeed the healthy brain. Young and old researchers alike debated well into the night around packed posters, where patience paid off for those trying to squeeze close enough to see the data up-close, and co-authors tag-teamed to present their findings for the umpteenth time.
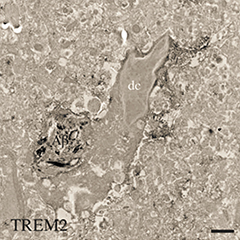
Dark Side of Microglia.
A newly identified type of microglia (dc) that engulfs Aβ (black) and expresses TREM2 appears dark in electron micrographs. [Courtesy of Marie-Ève Tremblay.]
“I got the sense from this meeting that people want to work more closely together,” said Beth Stevens, Children’s Hospital, Boston, who co-organized the microglial symposium together with Richard Ransohoff from Biogen, Cambridge, Massachusetts. Stevens emphasized the importance of collaboration for a field just beginning to get traction. “We now know what we need to do. We have a road map, the tools, and the insight. We can work individually in our own labs, or work together and make much more rapid progress,” she told Alzforum. Bradley Hyman, Massachusetts General Hospital, noted the value of having meetings on these two topics run side by side. Hyman co-organized the neurodegeneration symposium together with Adriano Aguzzi, University of Zurich, and Ricardo Dolmetsch from Novartis Institutes for BioMedical Research, also in Cambridge. “The cross-talk was really valuable, especially given how genetics now emphasizes that microglia and astrocytes are not secondary players but more primary drivers of disease,” Hyman said.
Some dogmas fell at this meeting even as researchers navigated new waters. Marco Prinz, University of Freiburg, Germany, put the kibosh on the prevailing idea that only brain-resident microglia originate from the yolk sac early in development. He reported evidence that seemed to convince even the most die-hard fans that non-parenchymal macrophages such as perivascular, meningeal, and choroid plexus macrophages are in fact long-lived macrophages that derive from yolk-sac progenitors, rather than bone-marrow-derived cells that turnover rapidly. “You can throw the text books out,” noted Gary Landreth, Stark Neurosciences Research Institute at the Indiana University School of Medicine in Indianapolis. “I see nothing wrong with that data set,” he told Alzforum. Ransohoff agreed. “I was completely shocked by that, but I’ll survive,” he quipped. “I think this is a robust finding, at least in mice, and we don’t have a lot of evidence that people are any different from mice in this respect.”
In general, scientists at the meeting agreed that they need to learn much more about microglial function in the normal brain before they can begin to understand how these cells influence disease. From potassium channels that regulate how microglial branches move and survey the brain, to active enhancers and transcriptome profiles that characterize these cells, researchers appear poised to more rigorously characterize microglia than just a few short years ago.
They are also developing better culture systems. A recurring theme at Keystone was that microglia de-differentiate rapidly—within eight hours—once they are taken out of their normal brain environment. While this knowledge left scientists questioning the relevance of most existing in vitro studies, they also agreed that it presents a valuable opportunity to understand what makes microglia tick. “If we can get these cultured cells back to looking like they do in vivo, we may have learned truly something about the brain,” noted Christopher Glass, University of California, San Diego, who gave the keynote address at the microglia meeting.
Along that vein, several groups reported that they had succeeded in creating microglia from human induced pluripotent stem cells—a first for the field. One group reported that when co-cultured with astrocytes and neurons, these induced microglia adopt features of brain microglia, including processes that extend and retract. “There goes another dogma,” noted Stevens. “If you had asked me a week ago if we could make induced microglia in vitro I’d have said, ‘No.’ Now three groups have reported on it.” Studying these cultures may bring the field closer to understanding microglial in the brain, researchers agreed.
Astrocytes got plenty of love at this meeting, too. For one, Shane Liddelow from Ben Barres’ lab at Stanford University, California, reported on different activation states of reactive astrocytes. To the chagrin of some researchers who have come to question the M1 and M2 designations for microglial activation, which many believe is too simplistic a nomenclature, Liddelow called these A1 and A2. He said the A1 astrocytes associate with neurotoxicity. Liddelow reported being able to culture these astrocytes in vitro and has identified several markers that characterize them, exciting other researchers who now plan to look for them in autopsy samples.
Researchers also reported stark differences between male and female microglia. This apparently is true both developmentally and later in life, prompting a re-evaluation of age- and sex-related risk factors for disease. On another curious note, Marie-Ève Tremblay from Laval University, Quebec, described a new type of microglia that show up as being particularly dense under the electron microscope. While their origin and function have yet to be determined, these “dark” microglia appear to occur in disease states and reside near blood vessels, close to amyloid plaques in mouse models of AD. They express TREM2, a microglial cell-surface receptor and now much-studied AD risk factor.
On TREM2 itself, some reported that this receptor may be involved in inflammation, and that it is important for microglial activation, proliferation, and phagocytosis. Scientists have long puzzled about what ligands might activate this receptor, and at Keystone, evidence emerged that it binds ApoE, ApoJ, and low-density lipoprotein. TREM2 also binds Aβ when the latter forms a complex with LDL, according to one presentation, whereupon microglia take it up the peptide and degrade it. TREM2 variants that associate with disease seem less effective in this respect.
On the neurodegeneration side of the Keystone hallways, researchers noted commonalities among various neurodegenerative diseases, from the biophysical structure of amyloid-forming proteins right up to how they scupper cells, both cell-autonomously and otherwise. Scientists also showed new data on how novel therapeutics—from dual leucine zipper kinase inhibitors to zinc-finger translational repressors that can be tailored to shut off transcription of specific genes—may work across a spectrum of diseases. Check back in the coming days as Alzforum brings you details on some of the highlights of the joint meeting.—Tom Fagan
References
No Available References
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.