New Ideas for Alzheimer’s Treatment: What’s on Offer in 2015?
Quick Links
Researchers agree that a disease as complex as Alzheimer’s may require combination therapies, but so far, few trials have taken on this challenge. That may soon change. At the 7th Clinical Trials on Alzheimer’s Disease (CTAD) conference in Philadelphia November 20 to 22, an impassioned case for developing multiple investigational compounds together came from none other than Rusty Katz, who last year retired from the Food and Drug Administration (see Part 2 of this series). Also at CTAD, Jean-Marc Orgogozo of University Hospital Pellegrin, Bordeaux, France, presented one example of a combination treatment, though not of investigational drugs. He reported that a combination of two repurposed drugs, neither of which has any efficacy for AD on its own, improved cognition in AD patients over a 12-week trial. “I’m delighted to hear of the evolution in the FDA’s thinking,” Orgogozo said.
That said, his trial is the exception. For the most part, CTAD featured single therapies directed at a variety of targets. Taken together with an update on European trials of non-pharmacological interventions, the meeting showcased a field trying to implement calls to broaden therapeutic approaches beyond amyloid and tau. Below, a summary.
In collaboration with the pharmaceutical company Pharnext in Issy-les-Moulineaux, France, Orgogozo led the PLEODIAL I study to test PXT00864. This compound mixes low doses of acamprosate calcium, used to treat alcohol dependency, and the muscle relaxer baclofen, approved for spasticity. Both drugs tweak neurotransmission. Acamprosate is an analog of the neurotransmitter GABA, while baclofen activates the GABA-B receptor. Together, the drugs appear to suppress excitatory and boost inhibitory signaling, Orgogozo said. This would appear to be an opposite effect to idalopirdine, which is currently in Phase 3 (see Part 10 of this series). Orgogozo believes the PXT00864 combination may help restore balance to AD brains, which are prone to hyperexcitability (see Sep 2007 news story). In two different AD mouse models, the combination restored working memory and object recognition to wild-type levels, and kept neurons alive, Orgogozo reported. When tested separately, neither drug had any effect.
The Phase 2a PLEODIAL trial enrolled 30 people with mild AD. Each participant took one of two doses of PXT00864 for four weeks, switched to placebo for four more weeks, then returned to the drug for the remainder of the trial. This design increases the trial power because each participant acts as his or her own control. While on the drug, participants improved on the ADAS-Cog11, whereas they declined while on placebo, Orgogozo said. The researchers saw a trend toward improvement on tests of executive function as well, but the scores were more variable and did not reach significance, Orgogozo said. Patients tolerated the drug well, mainly reporting some dizziness and fatigue at the higher dose. The researchers are running an extension trial of PXT00864, and plan next to test three different doses of the drug in a six-month Phase 2 study. A poster presented by Pharnext scientists suggested that in 20 healthy, young, male volunteers, PXT00864 enhanced recovery from a temporary memory impairment induced by scopolamine, an antagonist of cholinergic transmission that is often used in memory research.

Resveratrol, spinning in vain to get into the brain?
While PXT00864 appears to act via neurotransmission, the polyphenol resveratrol may act through multiple different mechanisms. Resveratrol occurs naturally in foods such as red grapes and dark chocolate, and is selling briskly as a supplement. It has been variously reported to activate deacetylases known as sirtuins, which are believed to extend life; have antioxidant properties; and prevent amyloid deposition (see Jun 2010 news story; Sep 2013 news story). Several studies are testing this compound in people with cognitive impairment or dementia, and researchers recently reported that six months of resveratrol supplements improved memory and brain metabolism in a small trial of overweight, cognitively healthy older people (see Jun 2014 news story).
At CTAD, Scott Turner of Georgetown University, Washington, D.C., reported on the 12-month Phase 2 Alzheimer’s Disease Cooperative Study (ADCS) of resveratrol. In this trial, 120 AD patients at 20 ADCS sites started on 500 mg per day of synthetic resveratrol, gradually increasing to 2,000 mg per day. The compound reached maximum microgram concentrations in the blood within two hours and persisted for many hours, Turner reported. However, less than 1 percent of resveratrol and its metabolites entered the brain, with cerebrospinal fluid (CSF) concentrations topping out at the nanomolar level. Participants taking the supplement lost about one kilogram of weight during the study, but otherwise tolerated the compound well.
As with many recent trials, the researchers saw more movement on biomarkers than cognition or function. Only one clinical outcome reached significance, with the treatment group better able to perform activities of daily living than the placebo group. Turner noted that the trial was not powered to detect clinical improvement, and he did not expect to see change there. On the other hand, CSF Aβ40 stabilized in the treated group, while it dropped by about 14 percent in controls. In plasma, Aβ40 dropped by 6 percent in participants on resveratrol, but plummeted by 20 percent in controls, Turner reported. A similar trend occurred with Aβ42 Turner said. To him, the data suggest that resveratrol engaged its target.
The steep decline in Aβ40 in the control group over the course of one year was soon questioned by the audience. In AD patients, CSF Aβ levels normally bottom out around the time symptoms begin (see Jan 2012 news story; Jul 2012 news story), though Turner noted that one study reported continued decline as dementia worsened (Nitsch et al., 1995). Few studies have charted longitudinal Aβ changes in plasma, but what evidence does exist suggests that there are no consistent changes in these markers (see Donohue et al., 2014).
In the ADCS trial, people on resveratrol lost more brain volume than those on placebo, an effect that has been seen in several immunotherapy trials. Turner noted that resveratrol can dampen inflammation, which might explain the shrinkage.
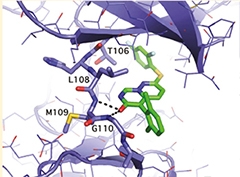
X-ray crystal structure of VX-745 bound to p38 MAPK alpha. [Image courtesy of Vertex Pharmaceuticals.]
A repurposed compound with anti-inflammatory properties appears headed for clinical trials. John Alam, who started the virtual biotech company EIP Pharma in Cambridge, Massachusetts, introduced the CTAD audience to VX-745, a small molecule that inhibits the mitogen-activated protein kinase, aka p38 MAPK alpha (see Duffy et al., 2011). This enzyme stimulates release of the inflammatory cytokines TNFα and IL-1β from microglia (see Yasuda et al., 2011). In animal models, VX-745 appears to shift the balance from pro-inflammatory M1 to phagocytic M2 microglia, in the process improving mitochondrial function, synaptic transmission, and memory, Alam said. In 2-year-old Tg2576 mice treated with 3 mg/kg for two weeks, amyloid plaque load dropped by half, perhaps through enhanced microglial clearance, Alam suggested. This research was done at the CRO Charles River Laboratories in Kuopio, Finland.
Because these mice develop little neuroinflammation, the researchers tested the drug on aged rats, which develop more. Three weeks of treatment at the highest dose tested, 4.5 mg/kg, lowered IL-1β levels while the postsynaptic marker PSD95 trended higher. The drug boosted cognition, with the rats that received 1.5 mg/kg faring best and performing like youngsters in the Morris water maze, Alam said (see Alam, 2014).
VX-745 is an old drug that was discovered at Vertex Pharmaceuticals, where Alam worked at the time (see Haddad, 2001). It was being tested for rheumatoid arthritis, but Vertex discontinued this work about a decade ago, reportedly in part due to adverse effects in the central nervous system (see news release).
Patients in those studies took 250 mg twice/day, which according to Alam equated to 10 mg/kg in rats. Alam has filed for a patent on using VX-745 for Alzheimer’s disease. He will first conduct a Phase 2a trial of VX-745 in patients with prodromal AD at VU Medical Center in Amsterdam, with Philip Scheltens of VU University Medical Center, Amsterdam, as principal investigator; this trial will start screening patients in early 2015. The second trial, to be done in the United States, is still being planned. Participants would take either 125 or 40 mg twice daily. The former is equivalent to the dose that suppressed inflammation in rats, the latter to the dose that best sharpened their cognition. Alam noted that VX-745 preferentially enters the brain, reaching concentrations almost twice those in plasma.
Finding effective therapies is not all about new drugs. Sandrine Andrieu, an epidemiologist at INSERM, Toulouse University, France, told CTAD attendees that several small studies of diet, exercise, and cognitive stimulation have shown promise in bolstering cognition or delaying AD, but need validation in large, biomarker-supported studies. The European Dementia Prevention Initiative (EDPI) coordinates three large prevention trials (see Jun 2012 conference story; Sep 2012 news story), each of which enroll more than 1,000 older adults at risk of dementia and provide intensive health and cardiovascular treatments over the course of several years. One of these, the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), recently reported better cognition in seniors who followed its plan (see Jul 2014 conference story).
The findings have stoked interest in this area. EDPI has begun a pilot study called Healthy Aging Through Internet Counseling in the Elderly (HATICE) to see if information given over the Internet can help healthy older people ward off dementia. Another prevention study, Do-Health, just finished recruiting 2,000 adults 70 and older from seven European cities. The trial will test whether Vitamin D, omega-3 fatty acids, and exercise can help maintain cognitive performance.
These trials have limitations, Andrieu noted. Volunteers tend to be highly educated and may not represent the general population. Because they score quite well on cognitive tests at baseline, there may not be much room for improvement. Screening volunteers for brain amyloid deposition could enrich for those at higher risk, she suggested. Prevention trials might also need to run longer than the current ones do to demonstrate an effect. For example, participants in the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study improved over the control group only after five years or more (see Rebok et al., 2014). Long trials are expensive and suffer high attrition, however. In addition, researchers should keep in mind that different interventions may work better for different groups of people, Andrieu said.
Despite these challenges, Andrieu stressed the importance of developing these approaches along with pharmaceutical options. “Even a small individual benefit can have a huge effect at the population level,” she said.— Madolyn Bowman Rogers
References
News Citations
- Rusty Unleashed: Forget Disease Modification, Go for Big Effect
- New Treatments for Alzheimer’s Behavioral Symptoms on Horizon
- Do "Silent" Seizures Cause Network Dysfunction in AD?
- One Small Molecule Detoxifies Different Forms of Aβ
- Brain-Specific Sirtuin Expression Delays Aging in Mice
- Resveratrol Improves Memory in Overweight Adults
- Research Brief: More Evidence That CSF Aβ Changes Precede AD
- Paper Alert: DIAN Biomarker Data Show Changes Decades Before AD
- Stockholm: Science Smorgasboard at Biannual Meeting
- Europe Asks If Reforming Health Habits Can Prevent Dementia
- Healthy Lives, Healthy Minds: Is it Really True?
Therapeutics Citations
Research Models Citations
Paper Citations
- Nitsch RM, Rebeck GW, Deng M, Richardson UI, Tennis M, Schenk DB, Vigo-Pelfrey C, Lieberburg I, Wurtman RJ, Hyman BT. Cerebrospinal fluid levels of amyloid beta-protein in Alzheimer's disease: inverse correlation with severity of dementia and effect of apolipoprotein E genotype. Ann Neurol. 1995 Apr;37(4):512-8. PubMed.
- Donohue MC, Moghadam SH, Roe AD, Sun CK, Edland SD, Thomas RG, Petersen RC, Sano M, Galasko D, Aisen PS, Rissman RA. Longitudinal plasma amyloid beta in Alzheimer's disease clinical trials. Alzheimers Dement. 2014 Oct 6; PubMed.
- Duffy JP, Harrington EM, Salituro FG, Cochran JE, Green J, Gao H, Bemis GW, Evindar G, Galullo VP, Ford PJ, Germann UA, Wilson KP, Bellon SF, Chen G, Taslimi P, Jones P, Huang C, Pazhanisamy S, Wang YM, Murcko MA, Su MS. The Discovery of VX-745: A Novel and Selective p38α Kinase Inhibitor. ACS Med Chem Lett. 2011 Oct 13;2(10):758-63. Epub 2011 Jul 28 PubMed.
- Yasuda S, Sugiura H, Tanaka H, Takigami S, Yamagata K. p38 MAP kinase inhibitors as potential therapeutic drugs for neural diseases. Cent Nerv Syst Agents Med Chem. 2011 Mar 1;11(1):45-59. PubMed.
- Alam J. Effects on Cognition of a Selective P38MAPK Alpha Inhibitor in Aged Rats. Alzheimers Dement. 2014 Jul;10(4, Suppl);P922.
- Haddad JJ. VX-745. Vertex Pharmaceuticals. Curr Opin Investig Drugs. 2001 Aug;2(8):1070-6. PubMed.
- Rebok GW, Ball K, Guey LT, Jones RN, Kim HY, King JW, Marsiske M, Morris JN, Tennstedt SL, Unverzagt FW, Willis SL. Ten-Year Effects of the Advanced Cognitive Training for Independent and Vital Elderly Cognitive Training Trial on Cognition and Everyday Functioning in Older Adults. J Am Geriatr Soc. 2014 Jan 13; PubMed.
External Citations
Further Reading
Papers
- Shineman DW, Alam J, Anderson M, Black SE, Carman AJ, Cummings JL, Dacks PA, Dudley JT, Frail DE, Green A, Lane RF, Lappin D, Simuni T, Stefanacci RG, Sherer T, Fillit HM. Overcoming obstacles to repurposing for neurodegenerative disease. Ann Clin Transl Neurol. 2014 Jul;1(7):512-8. Epub 2014 Jun 24 PubMed.
News
- Sirtuin Bests Huntingtin in Tug-of-War for Neural Survival
- Sirtuins’ Status Less Certain as Study Questions Longevity Effect
- Research Brief: SIRTs Keep Brain Minty Fresh
- Dietary Resveratrol Makes Little Difference to Health
- Combination Drug Trials: Time to Open a New Front in AD?
- First Stab at Combination Therapy Yields Additive Effect
- Building a Roadmap for Shared Combination Drug Trials
- Combination Trial Debate Energizes Keystone Symposium
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.