Hats Off to ApoE—Key to Formation of Functional Amyloid
Quick Links
See Part 5 and Part 6 of this series.
Most amyloid fibrils in the human body are toxic, but a select few perform important functions. What sets them apart? At the Society for Neuroscience annual meeting, held November 15 to 19 in Washington, D.C., Guillaume van Niel from the Institut Curie in Paris suggested that it could be ApoE. The lipoprotein concentrates on vesicles where fragments of the premelanosome protein (PMEL) congregate, and seems key to their orderly fibrillization. PMEL amyloid serves as a scaffold for the pigment melanin. Van Niel said the findings may help scientists understand how Aβ interacts with ApoE4, the strongest genetic risk factor for Alzheimer’s disease.
Pigment cells create PMEL fragments in a way that parallels processing of the amyloid precursor protein (APP) (see Nov 2008 news story). PMEL traffics from the Golgi to the plasma membrane and then to endosomes. There, BACE2 sheds the large extracellular domain of PMEL into the lumen of the endosome, while γ-secretase further processes what's left (see image below). In the case of PMEL the shed portion (the equivalent of sAPPβ) goes on to be cleaved by other proteins, yielding fragments that make amyloid fibrils on the intraluminal vesicles. The amyloid scaffolds are then shunted off to melanosomes, where they concentrate melanin.
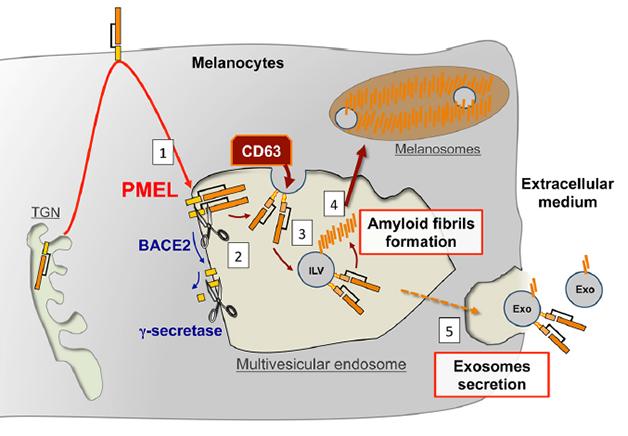
Parallel Processing? PMEL follows a similar trafficking and processing path to APP, and forms fibrils on intraluminal vesicles inside multivesicular bodies. [Image courtesy of Guillaume van Niel in Rajendran et al., 2014 J Neurosci.]
To figure out what makes the PMEL fibril harmless, van Niel took a close look at where they form. He snap-froze exosomes—intraluminal vesicles that had been expelled from the cells (see Part 5 and Part 6 of this series). Viewed up-close with an electron microscope, he noticed a cap-like structure on the outer membrane of these vessels. The cap’s cylindrical structure comprised multiple layers separated by 3.5 nm each. After two years of trying to figure out what the cap was, van Niel spotted a similar structure in an electron microscope image of a lipoprotein particle—multiple layers separated by 3.5 nm. Using a type of mass spectroscopy, he and colleagues checked if exosome caps contained any apolipoproteins. There was indeed one enriched in exosomes sporting caps compared to those without—none other than ApoE. “We got excited because a variant of ApoE is the primary genetic risk factor for sporadic AD,” said van Niel. Given the parallels between APP and PMEL processing, this finding could offer a clue about how ApoE interacts with APP’s fragments, he said.
To see if the ApoE cap interacted with the amyloid fragment of PMEL, van Niel used co-immunoprecipitation and found that the two bound each other. Electron microscopy revealed that this happened on the surface of the intraluminal vesicles inside multivesicular bodies of pigment cells. If the scientists depleted ApoE mRNA from the cells, the PMEL did not fibrillize. Instead, it formed unstructured aggregates in the lumen of the multivesicular body. The same organization defects plagued pigment cells from mice genetically deficient in ApoE. This suggested that ApoE is necessary to form the PMEL fibril on the vesicle outer membrane.
Scientists at the conference found this discovery extremely interesting. “What makes it so striking is that the cell traffics and cleaves PMEL similarly to APP,” said Gunnar Gouras, Lund University, Sweden. He thought it might help researchers understand the relationship between Aβ and ApoE, saying “We have been slow to understand how ApoE ties into Alzheimer's." Others remained to be convinced. “I was intrigued by this presentation, though it is hard to understand how it could relate to Alzheimer’s disease,” said Martin Ingelsson, Uppsala University, Sweden.
Van Niel said his findings suggest ApoE has a natural affinity for amyloid-forming peptides, and tends to fibrillize them. Since neurons do not normally produce ApoE, it only encounters their Aβ peptides after they leave the cell, whereupon ApoE may nucleate formation of fibrils (see image below).
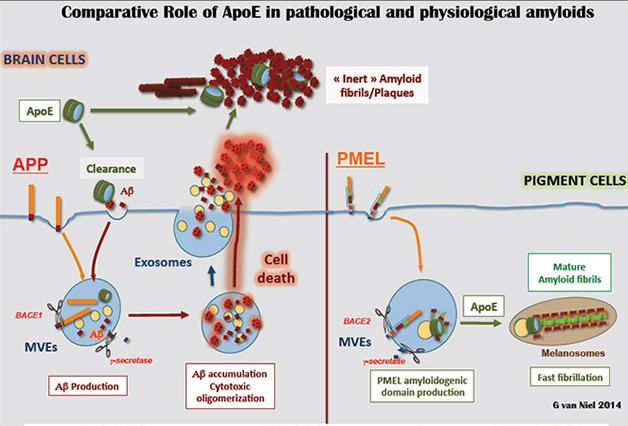
Put a Cap on It: In pigment cells (right), ApoE caps (green) nucleate ordered PMEL fibrils on intraluminal vesicles (yellow). In the brain (left), ApoE may encounter Aβ peptides (red) outside the cell. [Image courtesy of Guillaume van Niel.]
Gouras looked at this a slightly different way. He speculated that ApoE does make its way into neuronal endosomes in AD. Neurons might take up ApoE from glial cells, or they may produce it themselves after injury, he said (see Xu et al., 2006). If so, then ApoE and Aβ may interact inside the cells after all.
Ingelsson recommended testing exosomes from AD-related cell systems for ApoE. Lawrence Rajendran, University of Zurich, Switzerland, and van Niel together are looking for ApoE caps on exosomes from astrocytes. If there, they want to know if they help Aβ form fibrils, and whether caps made from the different isoforms of ApoE—ApoE2, or ApoE3, or ApoE4—do this differently.—Gwyneth Dickey Zakaib
References
News Citations
- Exosomes: The FedEx of the Nervous System?
- Exosomes: Purveyors of Neurodegenerative Disease?
- Let There Be Sunlight—Melanogenesis as a Model for AD?
Paper Citations
- Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, Huang Y. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci. 2006 May 10;26(19):4985-94. PubMed.
Further Reading
Papers
- Liao F, Hori Y, Hudry E, Bauer AQ, Jiang H, Mahan TE, Lefton KB, Zhang TJ, Dearborn JT, Kim J, Culver JP, Betensky R, Wozniak DF, Hyman BT, Holtzman DM. Anti-ApoE antibody given after plaque onset decreases Aβ accumulation and improves brain function in a mouse model of Aβ amyloidosis. J Neurosci. 2014 May 21;34(21):7281-92. PubMed.
- Rochin L, Hurbain I, Serneels L, Fort C, Watt B, Leblanc P, Marks MS, De Strooper B, Raposo G, van Niel G. BACE2 processes PMEL to form the melanosome amyloid matrix in pigment cells. Proc Natl Acad Sci U S A. 2013 Jun 25;110(26):10658-63. PubMed.
- Watt B, van Niel G, Raposo G, Marks MS. PMEL: a pigment cell-specific model for functional amyloid formation. Pigment Cell Melanoma Res. 2013 May;26(3):300-15. Epub 2013 Feb 19 PubMed.
- Watt B, van Niel G, Fowler DM, Hurbain I, Luk KC, Stayrook SE, Lemmon MA, Raposo G, Shorter J, Kelly JW, Marks MS. N-terminal domains elicit formation of functional Pmel17 amyloid fibrils. J Biol Chem. 2009 Dec 18;284(51):35543-55. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.