Toxic Stew of Aβ Dimers Hides Out in Human Plaques
Quick Links
The question of which Aβ species are toxic in Alzheimer’s disease is often asked, and the answer has been maddeningly elusive. At the Society for Neuroscience annual meeting in San Diego November 3–7, the lab of Dominic Walsh, Brigham and Women’s Hospital, Boston, took a fresh stab at it with new data presented by Wei Hong. The scientists once again nominated Aβ dimers as the toxic form of the peptide found in amyloid plaques. The difference this time around? Not one, but many pairs of different Aβ monomers linked by covalent bonds. These dimers are damaging to neurons. “This is the first-ever definitive proof of a toxic dimer, and the first identification of a specific cross-link that holds monomers together,” Walsh told Alzforum.
- Amyloid plaques contain cross-linked Aβ dimers toxic to neurons.
- Mass spectrometry reveals a diverse mix of dimers.
- A covalent bond links monomers into dimers.
When Aβ from aqueous brain extracts is analyzed by SDS-PAGE, a common method of separating proteins on a gel, two prominent bands emerge—a 4-kDa band consisting of Aβ monomers, and a 7-kDa band of unknown origin. The monomers seem harmless to neurons in cell and slice culture, but the 7-kDa band is toxic. Walsh, now in collaboration with Kaj Blennow, Henrik Zetterberg, Erik Portelius, and Gunnar Brinkmalm, all of the University of Gothenburg, Sweden, has spent the better part of a decade trying to pin down the molecular identity of that 7-kDa band. In 2008, he reported that they were likely dimers of Aβ (Nov 2007 conference news; Jun 2008 news). However, to show that definitively, he would need to do mass spectrometry, which proved to require an impossibly large sample of aqueous oligomers isolated from human brain.
In the present study, Hong and colleagues got around this problem by using material isolated from Aβ plaques, which litter AD brains and are a rich source of Aβ. The researchers used six autopsied brains provided by Matt Frosch at Massachusetts General Hospital. Dissolving the plaques in formic acid released 4- and 7-kDa bands that resembled those found in aqueous extracts. Once again the material in the 4-kDa was harmless to neurons, but the contents of the 7-kDa band disrupted neurites and blocked long-term potentiation in neurons derived from induced pluripotent stem cells. This time around, the researchers had enough 7-kDa material in hand for mass spectrometry that would determine the structure of the neurotoxic material.
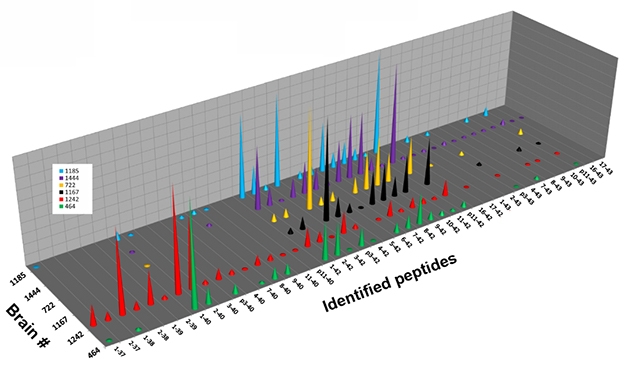
Monomer Diversity. Six individual brains (separate colors) contained different fragments of Aβ monomer, isolated from the 4-kDa band of solubilized plaques. [Image courtesy of Dominic Walsh.]
In the 4-kDa band, liquid chromatography followed by mass spectrometry revealed more than 35 different monomeric Aβ species, in different abundances in the six brains examined (see image above). Analyzing the 7-kDa band in the same way revealed a broad mix of peaks, whose masses were consistent with diverse Aβ heterodimers. The mix comprised many combinations of Aβ37 to Aβ42, the 11 most abundant of which were 1-37 plus 1-38, 1-38 plus 1-38, 1-38 plus 1-39, 1-38 plus 1-40, 1-39 plus 1-40, 1-40 plus 1-40, 2-38 plus 1-40, 2-40 plus 1-38, 2-40 plus 1-40, 1-40 plus 1-42, and 1-42 plus 1-42.

Dimer Diversity. The 7-kDa fraction of Aβ isolated from human AD plaques contains a mix of species whose component masses are consistent with Aβ dimers. [Image courtesy of Dominic Walsh.]
To determine where these dimers were cross-linked, the researchers digested the 7-kDa material with trypsin/LysC, which cleaves after arginine and lysine residues. They then used mass spec-mass spec to analyze the fragments. In addition to fragments that would be anticipated from Aβ lacking a cross-linking covalent bond, they also detected a fragment encompassing residues 1–5 and 17–28 with a cross-link between Asp1 and Glu22. That suggested the presence of dimers held together by a covalent bond.
Walsh said that so far his group has identified only one specific cross-link. Others likely exist given that many Aβ alloforms lack the Asp1 amino acid, he said. “This is likely the tip of the iceberg,” he told Alzforum.
The dimers in the 7-kDa band represent one toxic form of Aβ, though Walsh was careful to note that dimers are not the only one. Understanding which dimers are most toxic could allow researchers to target them specifically and perhaps protect neurons better. For instance, anti-Aβ antibodies that recognize covalent dimers could have more therapeutic benefit than antibodies that don’t.
The 7-kDa band isolated from the 7PA2 Chinese hamster ovary cell line, which overproduces Aβ42 due to a V717F mutation in APP, was previously reported to contain N-terminally extended fragments of Aβ, as well as large Aη fragments that are products of an alternative APP cleavage (May 2015 news; Aug 2015 news). However, in the 7-kDa fraction isolated from human brains, mass spec evidence of a covalent bond between trypsin-digested Aβ fragments, and no evidence of Aη, suggests that the toxic species in these extracts are instead dimers of Aβ, Portelius wrote to Alzforum.
“The analytical study done is exceptional and among the best mass spectrometry data to date,” wrote Randall Bateman, Washington University School of Medicine, St. Louis, to Alzforum. “It clearly identifies several novel cross-linked amyloid-β species in bands that could be oligomers.” He raised several questions, namely which of the dimers identified here are toxic in Alzheimer’s disease, and how to control for artifacts created by homogenization and extraction techniques. Bateman also wondered how these cross-linked species relate to various stages of AD, and how they cause toxicity in vivo.
“I think it’s likely that these are real, covalently linked dimers.” wrote David Brody, Uniformed Services University of the Health Sciences, Bethesda, Maryland, to Alzforum. “The big question is whether they exist in the soluble phase and at what concentration.”
Walsh plans to create antibodies specific for the Asp1-Glu22 cross-link. With them, he could determine the abundance of these dimers in native aqueous brain extracts and whether they relate to the presence and severity of the disease. He and his Swedish collaborators also want to explore whether these dimers could serve as fluid biomarkers for Alzheimer’s disease.—Gwyneth Dickey Zakaib
References
News Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.