Sparking the Brain May Enhance Plasticity, Memory
Quick Links
Two new papers add weight to the idea that brain stimulation benefits both plasticity and memory. In the October 25 JAMA Psychiatry, researchers led by Tarek Rajji at the Centre for Addiction and Mental Health, Toronto, reported that plasticity in the dorsolateral prefrontal cortex falters in Alzheimer’s disease, and that this deficit correlates with poorer working memory. Transcranial magnetic stimulation, which uses magnetic coils at the scalp to induce currents in neurons, strengthened those synapses, though not as robustly as in age-matched healthy controls. In the second paper, published in the October 24 eLife, researchers led by Itzhak Fried at the University of California, Los Angeles, reported that microelectrodes implanted in the brain to stimulate the entorhinal cortex in epilepsy patients improved their performances on a facial-recognition task.
- Neural plasticity falters in the dorsolateral prefrontal cortex in AD.
- Transcranial magnetic stimulation strengthens DLPFC synapses.
- Tiny electrodes implanted deep in the brain improve memory in epilepsy.
“Both of these papers are exciting because they show that circuits in the brain mediating memory can be influenced using stimulation,” said Andres Lozano, University Health Network, Toronto. That normal memory and impaired synapse strength can both be enhanced lends credence to the idea that stimulation could help improve function where memory is impaired, he told Alzforum. While other researchers were intrigued by the scientific findings, they cautioned that this is still a long way from clinical application.
Rajji and colleagues focused on the DLPFC because it’s an area impaired early in AD (Battaglia et al., 2007; Kaufman et al., 2012). Scientists had previously reported impaired plasticity in the motor cortices of AD patients, but no one had yet assessed the DLPFC.
First author Sanjeev Kumar and colleagues used a paradigm called paired associative stimulation (PAS) to induce long-term potentiation (LTP), a measure of synaptic strengthening, in the DLPFC of 32 AD patients and 16 age-matched controls. This involves giving two pulses in quick succession—one in the brain preceded by one in another area of the body. The idea is that the first pulse strengthens the response to the second, which the subject learns to anticipate. In this case, Kumar electrically stimulated the right median nerve of the wrist, while using transcranial magnetic stimulation (TMS) to stimulate the contralateral DLPFC. This procedure triggers both pre- and postsynaptic action potentials to induce LTP, the theory goes, and has been used to induce plasticity of the motor cortex (Stefan et al., 2000). Both before and after PAS-LTP, Kumar and colleagues measured the cortical-evoked activity induced by TMS in the DLPFC using electroencephalography (EEG). If the cortical-evoked activity increased, that would indicate a strengthening of synaptic activity. The researchers also assessed working memory, which relies on the DLPFC, using an N-back test. This is when participants indicate whether a letter of the alphabet presented to them was the same or different than the one seen just prior (1-back), or the one prior to that (2-back).
Both the AD and control groups demonstrated heightened cortical activity after PAS, suggesting that the DLPFC could undergo synapse strengthening, even in AD patients. However, they experienced only half the strengthening as controls, suggesting plasticity wanes in the DLPFC in AD. Also, weaker plasticity in the DLPFC correlated with worse working memory. “This work suggests that there is hope of enhancing plasticity in AD patients’ frontal lobes to improve their memory,” wrote Rajji to Alzforum. He next plans to apply this paradigm in patients with mild cognitive impairment to see if they, too, show early signs of reduced DLPFC plasticity.
Joel Voss, Northwestern University, Chicago, cautioned that the authors didn’t test other areas of the brain to see if plasticity was impaired there, as well. “The findings could reflect brain-wide plasticity problems in AD, rather than a selective issue in the DLPFC,” he suggested. He also pointed out that the authors calibrated their TMS signal using responses in the motor cortex, which may poorly reflect stimulation of the DLPFC in AD because atrophy varies across the brain in this disease. Hence, Voss said the TMS intensity could have been weaker or more variable in the AD DLPFC than in controls, and that alone could account for the difference in plasticity. Rajji acknowledged these points but noted that they measured the difference between pre- and post-PAS-evoked activity, rather than just the magnitude of the response, and both were measured with the same TMS field. Further, the association with impaired working memory suggests that the DLPFC plasticity deficit is not an artifact of distance to the TMS coil, but a real synaptic problem, he said.
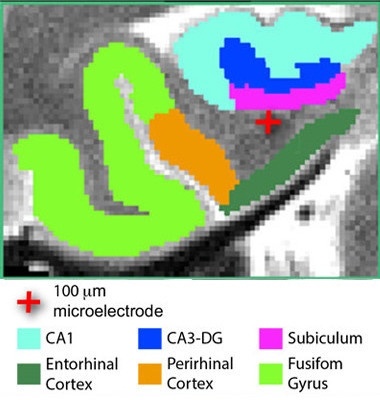
Focused stimulation.
A tiny electrode (red cross) stimulates the entorhinal area of an epilepsy patient. [Courtesy of Titiz et al., 2017, eLife.]
Co-first authors of the eLife paper Ali Titiz, Michael Hill, and Emily Mankin proposed another way to improve LTP. In their study, they tested whether focused stimulation with microelectrodes could improve facial recognition—and presumably LTP—in 13 people who were undergoing surgery for epilepsy. The researchers wanted to use a more targeted form of stimulation and physiologically relevant pattern than typically applied in deep-brain stimulation. They implanted tiny, 0.1mm diameter microelectrodes near the entorhinal cortex (ERC) to deliver pulses directly in a theta frequency pattern, typical of the slow-wave electrical activity exhibited by neurons of the hippocampus. This pattern was previously shown to elicit LTP in hippocampal slices from rats, as well as LTP in people when delivered by TMS (Larson et al., 1986; Suppa et al., 2016).
After the electrodes were implanted, patients were monitored for a one- to two-week observation period, then underwent multiple memory test sessions. For each, patients first underwent a learning phase, where they viewed about 30 images of faces. For half of these images, patients were first given a one-second burst of electricity in a 5 Hz theta frequency. For the other half, the electrode stayed silent. During the following recall phase, participants saw the same images interspersed with similar, but nonidentical “lure” images. Subjects indicated which image they had seen before and which were new. Titiz and colleagues counted how many images were correctly identified.
It turned out that subjects better remembered the images learned immediately after a theta burst, but only if it was given to the right hemisphere. Pulses in the left hemisphere made no difference. This makes sense given that previous studies assigned facial recognition capability to the right hippocampus (Haxby et al., 1996). The results imply that theta-burst stimulation delivered to the entorhinal area via microelectrodes enhances LTP and improves memory in epilepsy, wrote the authors, though there is no direct evidence for a change in LTP.
“The authors use new technology to show that capitalizing on the natural physiology of the targeted area has a beneficial effect on its function,” said Voss. Researchers looking to stimulate the brain to improve function need to account for how brain areas normally operate with each other and use technologies that exploit that, he said. However, he noted the huge gulf between the findings and practical application, given that these results came from carefully controlled experimental conditions. It’s not clear if or how microstimulation would benefit everyday memory, he said.
Lozano commented that it is unclear whether microelectrodes are preferable to the much larger electrodes used in deep-brain stimulation. Researchers may want to tailor the type of stimulation for each patient, he said. It would be great to test both in the same brain, at different times of day, and using different patterns of stimulation to find out which protocols are ideal, he said. As with many AD therapies proposed to date, a stimulation paradigm would be best applied early in disease, before neuronal and network deterioration, he added.
Abid Hussaini, Columbia University Medical Center, New York, wondered if there might be a downside. “I was surprised that stimulation in such a small region can have a profound effect on behavior,” he said. In a previous study, he and Karen Duff, also at Columbia, found that increasing neuronal activity in the hippocampus or ERC led neurofibrillary tangles of tau to accumulate in those respective locations in a mouse model of tauopathy (Jun 2016 news). He wondered if this type of stimulation could accelerate disease in AD patients.—Gwyneth Dickey Zakaib
References
News Citations
Paper Citations
- Battaglia F, Wang HY, Ghilardi MF, Gashi E, Quartarone A, Friedman E, Nixon RA. Cortical plasticity in Alzheimer's disease in humans and rodents. Biol Psychiatry. 2007 Dec 15;62(12):1405-12. PubMed.
- Kaufman LD, Pratt J, Levine B, Black SE. Executive deficits detected in mild Alzheimer's disease using the antisaccade task. Brain Behav. 2012 Jan;2(1):15-21. PubMed.
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000 Mar;123 Pt 3:572-84. PubMed.
- Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986 Mar 19;368(2):347-50. PubMed.
- Suppa A, Huang YZ, Funke K, Ridding MC, Cheeran B, Di Lazzaro V, Ziemann U, Rothwell JC. Ten Years of Theta Burst Stimulation in Humans: Established Knowledge, Unknowns and Prospects. Brain Stimul. 2016 May-Jun;9(3):323-335. Epub 2016 Jan 27 PubMed.
- Haxby JV, Ungerleider LG, Horwitz B, Maisog JM, Rapoport SI, Grady CL. Face encoding and recognition in the human brain. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):922-7. PubMed.
Further Reading
Papers
- Xia F, Yiu A, Stone SS, Oh S, Lozano AM, Josselyn SA, Frankland PW. Entorhinal Cortical Deep Brain Stimulation Rescues Memory Deficits in Both Young and Old Mice Genetically Engineered to Model Alzheimer's Disease. Neuropsychopharmacology. 2017 Dec;42(13):2493-2503. Epub 2017 May 25 PubMed.
- Lozano AM, Fosdick L, Chakravarty MM, Leoutsakos JM, Munro C, Oh E, Drake KE, Lyman CH, Rosenberg PB, Anderson WS, Tang-Wai DF, Pendergrass JC, Salloway S, Asaad WF, Ponce FA, Burke A, Sabbagh M, Wolk DA, Baltuch G, Okun MS, Foote KD, McAndrews MP, Giacobbe P, Targum SD, Lyketsos CG, Smith GS. A Phase II Study of Fornix Deep Brain Stimulation in Mild Alzheimer's Disease. J Alzheimers Dis. 2016 Sep 6;54(2):777-87. PubMed.
Primary Papers
- Titiz AS, Hill MR, Mankin EA, M Aghajan Z, Eliashiv D, Tchemodanov N, Maoz U, Stern J, Tran ME, Schuette P, Behnke E, Suthana NA, Fried I. Theta-burst microstimulation in the human entorhinal area improves memory specificity. Elife. 2017 Oct 24;6 PubMed.
- Kumar S, Zomorrodi R, Ghazala Z, Goodman MS, Blumberger DM, Cheam A, Fischer C, Daskalakis ZJ, Mulsant BH, Pollock BG, Rajji TK. Extent of Dorsolateral Prefrontal Cortex Plasticity and Its Association With Working Memory in Patients With Alzheimer Disease. JAMA Psychiatry. 2017 Dec 1;74(12):1266-1274. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.