Retinal Plaques May Enable Noninvasive Screening for AD
Quick Links
The eye, with its direct connection to the brain, has long tantalized researchers with its potential to serve as a window into Alzheimer’s disease pathology. Some researchers have found amyloid plaques accumulating in the lens or retina, although other data conflicts. In the August 17 Journal of Clinical Investigation Insight, researchers led by Maya Koronyo-Hamaoui at Cedars-Sinai Medical Center, Los Angeles, presented new data from postmortem samples that support the existence of amyloid plaques in retinas from AD patients. The authors claim that these plaques can be detected in the eyes of living people using a curcumin-based probe and a retinal scanning procedure developed by NeuroVision Imaging, a biotech company in Sacramento that Koronyo-Hamaoui co-founded. In a small pilot study, retinas in AD patients gave off twice as much amyloid signal as did those in healthy controls.
The researchers are collaborating with other groups to validate the technology in larger cohorts, as well as to determine how early the signal arises and how it changes over the course of disease. Koronyo-Hamaoui believes retinal amyloid imaging could be a promising biomarker for early diagnostic screening, and might help track progression as well.
Other researchers noted that though these data are preliminary, the findings are encouraging and deserve further study. “The authors’ demonstration both from pathological examination of the retinas, plus finding curcumin-positive (presumably Aβ-positive) deposits in living patients, amply justifies larger and definitive clinical trials,” David Knopman at the Mayo Clinic in Rochester, Minnesota, wrote to Alzforum.
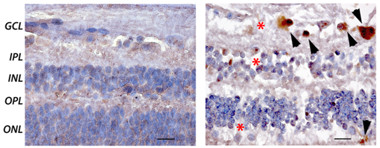
Plaques Accompany Degeneration.
Amyloid plaques (brown; arrowheads) and cell loss (nuclei blue; asterisks) occur in the ganglion cell layer and surrounding regions of retina in AD patients (right) but not controls (left). [Republished with permission of the American Society for Clinical Investigation.]
Researchers have been drawn to the eye because it is the most accessible central nervous system tissue, allowing for noninvasive screening. Reports of amyloid accumulation in the lens have been controversial (May 2013 news). Other groups considered the retina, which connects directly to brain through the optic nerve, a better bet for AD pathology. Koronyo-Hamaoui and colleagues first reported the retinal plaques in AD patients and mouse models of the disease (Koronyo-Hamaoui et al., 2011). Others have found similar retinal pathology in rodent models of AD (Alexandrov et al., 2011; Tsai et al., 2014). However, some papers report no evidence of amyloid plaques in retinas from AD patients or mouse models (Schön et al., 2012; Ho et al., 2013).
To definitively prove the existence of retinal plaques, first author Yosef Koronyo analyzed postmortem retinas from 23 AD patients and 14 healthy controls. The authors utilized numerous staining techniques, including immunostaining with anti-Aβ antibodies such as 6E10, 12F4, and 4G8, as well as using agents such as Congo red, thioflavin-S, and Gallyas silver stains that detect fibrillar Aβ deposits. With all these techniques, the authors consistently found clusters of Aβ deposits in the peripheral superior quadrant, or upper edge, of AD retinas. By contrast, central and lateral portions of the retina harbored very few deposits. Notably, the previous studies that failed to find retinal amyloid analyzed only these central regions.
The Aβ42-positive staining was about five times more extensive in AD patients than controls. Retinal staining correlated closely with cortical amyloid load in a subset of eight people who had donated both types of postmortem tissue. Retinal load particularly mirrored that of primary visual cortex, with a correlation coefficient ranging from 0.84 to 0.91, depending on the staining method.
Were these retinal deposits really plaques? The deposits had the appearance of typical neuritic plaques and ranged from diffuse to compact, the authors found. However, they were much smaller than brain plaques, having about one-sixth the diameter. Just as in brain parenchyma, the deposits were often found along blood vessels or in vessel walls. The authors analyzed some immunostained sections by transmission electron microscopy, and saw evidence of fibrils, protofibrils, and parallel β-sheets similar to the structures seen in brain plaques. Most of these deposits occurred in the ganglion cell layer of the retina, and were often accompanied by a loss of cells in this and nearby layers (see image above).
Other researchers called the pathology work a strength of the paper. Knopman found the evidence for the presence of amyloid plaques persuasive. However, Jochen Herms at the German Center for Neurodegenerative Diseases, Munich, was troubled by the extremely small size of these putative plaques, noting that in many cases they were smaller than cell nuclei. He wondered what the reason for such a size difference between retinal and parenchymal plaques might be.
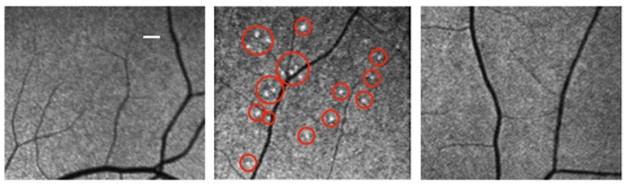
Lighting Up Retina. A curcumin-based probe detects fluorescent deposits (red circles) in an AD patient (middle), but not a healthy control (left) or a vascular dementia patient (right). [Republished with permission of the American Society for Clinical Investigation.]
To detect these plaques in living people, the authors turned to curcumin, a spice found in curry. Curcumin has structural similarities to Congo red and binds with high affinity to Aβ oligomers and fibrils (Dec 2004 news). The authors used a lipidated formulation that can be taken orally and passes into the retina, where it binds deposits and fluoresces. Sixteen participants took 4 g oral doses of the curcumin probe for two days. This translates to 1 g of curcumin each, about 10 times what aficionados of this spice take in supplements or in their Tikka Masala. Ten of the participants were diagnosed with mild to moderate AD, while the rest were healthy controls with a younger average age than the patients. The authors used a modified scanning laser ophthalmoscope to image participants’ retinas at baseline and two days. The raw images were extensively processed via automated algorithms to subtract baseline fluorescence, detect bright spots, and calculate a retinal amyloid index (RAI) based on the intensity of the signal.
With this method, the authors again saw clustered deposits in the superior quadrant of the retina in AD patients, but not controls (see image above). Among a subset of age-matched AD patients and controls, the former averaged about twice the RAI of the latter. The test cleanly separated most AD patients from controls, with only minor overlap between the groups, Koronyo-Hamaoui noted. The RAI score correlated with the number of fluorescent spots, as might be expected, but not with the age or MMSE scores of participants. Preliminary findings suggested the assay was selective for AD over other dementias and eye diseases. One participant with vascular dementia had no retinal signal, while two additional volunteers with age-related macular degeneration showed diffuse fluorescence around the macula near the center of the eye, but not in the superior quadrant.
The researchers do not yet have an explanation for why deposits primarily accumulate in this upper region. Koronyo-Hamaoui noted that the superior quadrant differs from the rest of the retina in vascular density, the choroid layer, and in the intensity of natural light that falls on it. In ongoing work, she is investigating whether this region clears amyloid more poorly than the rest of the eye does, allowing plaque to accumulate. Meanwhile, she is intrigued by the fact that numerous reports have found thinning of the nerve fiber layer in this region in AD patients (Lu et al., 2010; Kirbas et al., 2012; Kromer et al., 2014). Koronyo-Hamaoui and colleagues had also reported a loss of melanopsin-containing retinal ganglion cells in the superior quadrant of AD patients that correlated with amyloid accumulation there (La Morgia et al., 2016). Because these melanopsin-containing cells drive circadian rhythms, their loss could underlie some of the sleep disturbances and other visual deficits seen in AD patients, Koronyo-Hamaoui speculated (Gilmore et al., 1994; Trick et al., 1995; Risacher et al., 2013).
Other researchers called this initial trial promising, but noted that the method still has a long way to go to become a validated biomarker. Peter Nelson at the University of Kentucky, Lexington, suggested that the next step should be to test the sensitivity and specificity of the assay against established Aβ biomarkers, namely cerebrospinal fluid Aβ42 and amyloid PET. Such studies are ongoing, Koronyo-Hamaoui said. Shaun Frost at the Commonwealth Scientific and Industrial Research Organisation (CSIRO), Perth, Australia, has used the technology to scan more than 200 participants in the Australian Imaging, Biomarker & Lifestyle (AIBL) Flagship Study of Ageing. Preliminary results from this study found a close correlation between retinal fluorescence and PiB PET (Jul 2014 conference news). Frost is examining people at prodromal disease stages and following up with participants to see how the deposits change over time.
Meanwhile, in the United States, Allan Levey and Jim Lah at Emory University in Atlanta have added retinal scans using the NeuroVision technology to the suite of biomarkers they study at the Emory Alzheimer’s Disease Research Center and in the Emory Healthy Aging Study, which enrolls more than 3,000 people. So far, they have scanned more than 500 people who have also donated CSF samples, Levey told Alzforum. They plan to correlate the two biomarkers.
Other groups are also interested in finding ways to detect retinal amyloid. Researchers led by Melanie Campbell of the University of Waterloo, Canada, use polarized light to detect amyloid deposits in the retina. In 2016, she described a proof-of-concept trial showing that this method detected amyloid buildup as sensitively as established biomarkers (2016 press release). At the 2017 Alzheimer’s Association International Conference, held July 16–20 in London, Campbell reported finding amyloid plaques in postmortem AD retina that correlated with brain pathology.—Madolyn Bowman Rogers
References
News Citations
- Not Seeing Eye to Eye: Do Lenses Accumulate Aβ?
- Curry Ingredient Spices Things Up by Blocking Aβ Aggregation
- Alzheimer’s Disease: In the Eye of the Patient?
Paper Citations
- Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, Miller CA, Ko MK, Black KL, Schwartz M, Farkas DL. Identification of amyloid plaques in retinas from Alzheimer's patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage. 2011 Jan;54 Suppl 1:S204-17. PubMed.
- Alexandrov PN, Pogue A, Bhattacharjee S, Lukiw WJ. Retinal amyloid peptides and complement factor H in transgenic models of Alzheimer's disease. Neuroreport. 2011 Aug 24;22(12):623-7. PubMed.
- Tsai Y, Lu B, Ljubimov AV, Girman S, Ross-Cisneros FN, Sadun AA, Svendsen CN, Cohen RM, Wang S. Ocular changes in TgF344-AD rat model of Alzheimer's disease. Invest Ophthalmol Vis Sci. 2014 Jan 29;55(1):523-34. PubMed.
- Schön C, Hoffmann NA, Ochs SM, Burgold S, Filser S, Steinbach S, Seeliger MW, Arzberger T, Goedert M, Kretzschmar HA, Schmidt B, Herms J. Long-term in vivo imaging of fibrillar tau in the retina of P301S transgenic mice. PLoS One. 2012;7(12):e53547. PubMed.
- Ho CY, Troncoso JC, Knox D, Stark W, Eberhart CG. Beta-Amyloid, Phospho-Tau and Alpha-Synuclein Deposits Similar to Those in the Brain Are Not Identified in the Eyes of Alzheimer's and Parkinson's Disease Patients. Brain Pathol. 2013 May 29; PubMed.
- Lu Y, Li Z, Zhang X, Ming B, Jia J, Wang R, Ma D. Retinal nerve fiber layer structure abnormalities in early Alzheimer's disease: evidence in optical coherence tomography. Neurosci Lett. 2010 Aug 9;480(1):69-72. PubMed.
- Kirbas S, Turkyilmaz K, Anlar O, Tufekci A, Durmus M. Retinal Nerve Fiber Layer Thickness in Patients With Alzheimer Disease. J Neuroophthalmol. 2012 Oct 24; PubMed.
- Kromer R, Serbecic N, Hausner L, Froelich L, Aboul-Enein F, Beutelspacher SC. Detection of Retinal Nerve Fiber Layer Defects in Alzheimer's Disease Using SD-OCT. Front Psychiatry. 2014;5:22. Epub 2014 Feb 25 PubMed.
- La Morgia C, Ross-Cisneros FN, Koronyo Y, Hannibal J, Gallassi R, Cantalupo G, Sambati L, Pan BX, Tozer KR, Barboni P, Provini F, Avanzini P, Carbonelli M, Pelosi A, Chui H, Liguori R, Baruzzi A, Koronyo-Hamaoui M, Sadun AA, Carelli V. Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann Neurol. 2016 Jan;79(1):90-109. Epub 2015 Dec 18 PubMed.
- Gilmore GC, Wenk HE, Naylor LA, Koss E. Motion perception and Alzheimer's disease. J Gerontol. 1994 Mar;49(2):P52-7. PubMed.
- Trick GL, Trick LR, Morris P, Wolf M. Visual field loss in senile dementia of the Alzheimer's type. Neurology. 1995 Jan;45(1):68-74. PubMed.
- Risacher SL, Wudunn D, Pepin SM, Magee TR, McDonald BC, Flashman LA, Wishart HA, Pixley HS, Rabin LA, Paré N, Englert JJ, Schwartz E, Curtain JR, West JD, O'Neill DP, Santulli RB, Newman RW, Saykin AJ. Visual contrast sensitivity in Alzheimer's disease, mild cognitive impairment, and older adults with cognitive complaints. Neurobiol Aging. 2013 Apr;34(4):1133-44. PubMed.
External Citations
Further Reading
Primary Papers
- Koronyo Y, Biggs D, Barron E, Boyer DS, Pearlman JA, Au WJ, Kile SJ, Blanco A, Fuchs DT, Ashfaq A, Frautschy S, Cole GM, Miller CA, Hinton DR, Verdooner SR, Black KL, Koronyo-Hamaoui M. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer's disease. JCI Insight. 2017 Aug 17;2(16) PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
VU University Medical Center
With great interest, we read Koronyo’s paper on retinal amyloid visualization as a possible non-invasive biomarker in Alzheimer’s disease published in JCI insight and Alzforum’s coverage of it. Because of the huge and imminent interest in easily applicable and affordable biomarkers for AD, we too are studying in vivo and ex vivo retinal imaging in the Amsterdam Dementia Cohort of the VU University Medical Center (VUmc) Alzheimer Center in cooperation with the Dutch Brain Bank, ophthalmology department AMC and VUmc and Laserlab of the VU University. We were impressed by the large and comprehensive body of work described in Koronyo’s paper. In their postmortem work, they present both flat-mount techniques and cross sections to cover a large part of the retina and assess amyloid distribution over retinal layers with immunohistochemistry (amyloid antibodies), fluorescent techniques, and histochemical staining methods, such as Gallyas and Congo red (figure 1). Other labs, however, report absence of retinal amyloid. We therefore would like to discuss some methodological issues while interpreting the current data, before the results of this study can be translated into patients.
Firstly, in Koronyo’s paper, deposits with a range of size and morphologies are observed with different antibodies and stains (4G8, 6E10, 12F4, Gallyas, curcumin, Congo red) and aforementioned techniques and therefore present a complex picture. Flat mounts in figure 2, for example, show plaque-like structures, while cross sections in figure 4 show intracellular staining with the same antibody (12F4). That raises the question how different retinal deposits should be interpreted and how they relate to pathology in the brain. As the retina might physiologically process APP and amyloid differently than does the brain, retinal deposits may be different from those observed in the brain. Deposits shown could be reflective of (ab)normal APP processing in GCL, INL, or RPE cells (Ratnayaka et al., 2015) or could be true pathological deposits. Additional research is warranted to characterize the exact nature of retinal deposits in AD. Finally, it should be noted that all used Aβ antibodies have affinity for APP in higher concentrations (1:150 in this case). Intracellular inclusions, like the ones seen in figure 4, could also be interpreted as intracellular APP upregulation instead of extracellular amyloid deposits.
In conclusion, while we share the enthusiasm for the current findings and agree with the importance of identifying amyloid plaques outside of the brain as a possible biomarker, the postmortem findings of the current study need to be confirmed by other independent groups. We draw attention to the fact that groups from Johns Hopkins (Ho et al., 2014), Harvard (Williams et al., 2017), and the German Center for Neurodegenerative Diseases, Munich (Schön et al., 2012), were unable to do so before. We call for sharing exact protocols on tissue processing (flat-mount techniques and cross sections) and staining methods in order to robustly elucidate the presence of neuropathological hallmarks in retinas of AD patients. By cooperatively applying these protocols in independent labs, we together can eliminate effects of local variations in tissue processing and staining.
On behalf of the multidisciplinary I-READ team, Amsterdam, Tjado Morrema, Aleid van de Kreeke, Oleg Nadyarnykh, Jeroen Hoozemans, Frank Verbraak, Annemieke Rozemuller, Arthur Bergen, Philip Scheltens, Femke Bouwman, and Johannes de Boer.
References:
Ratnayaka JA, Serpell LC, Lotery AJ. Dementia of the eye: the role of amyloid beta in retinal degeneration. Eye (Lond). 2015 Aug;29(8):1013-26. Epub 2015 Jun 19 PubMed.
Ho CY, Troncoso JC, Knox D, Stark W, Eberhart CG. Beta-Amyloid, Phospho-Tau and Alpha-Synuclein Deposits Similar to Those in the Brain Are Not Identified in the Eyes of Alzheimer's and Parkinson's Disease Patients. Brain Pathol. 2013 May 29; PubMed.
Williams EA, McGuone D, Frosch MP, Hyman BT, Laver N, Stemmer-Rachamimov A. Absence of Alzheimer Disease Neuropathologic Changes in Eyes of Subjects With Alzheimer Disease. J Neuropathol Exp Neurol. 2017 May 1;76(5):376-383. PubMed.
Schön C, Hoffmann NA, Ochs SM, Burgold S, Filser S, Steinbach S, Seeliger MW, Arzberger T, Goedert M, Kretzschmar HA, Schmidt B, Herms J. Long-term in vivo imaging of fibrillar tau in the retina of P301S transgenic mice. PLoS One. 2012;7(12):e53547. PubMed.
Banner Sun Health Research Institute
Readers should see comments made earlier this year on the same topic, i.e., my own comment regarding labs at Johns Hopkins and Massachussetts General Hospital that have not seen Aβ or tau pathology in the retina of AD subjects (see references below).
Our own lab failed to see Aβ or tau pathology in unpublished work on whole-mount AD and control retina. Histological evidence needs to be confirmed with biochemical analysis, e.g., western blot of retina.
References:
Ho CY, Troncoso JC, Knox D, Stark W, Eberhart CG. Beta-Amyloid, Phospho-Tau and Alpha-Synuclein Deposits Similar to Those in the Brain Are Not Identified in the Eyes of Alzheimer's and Parkinson's Disease Patients. Brain Pathol. 2013 May 29; PubMed.
Williams EA, McGuone D, Frosch MP, Hyman BT, Laver N, Stemmer-Rachamimov A. Absence of Alzheimer Disease Neuropathologic Changes in Eyes of Subjects With Alzheimer Disease. J Neuropathol Exp Neurol. 2017 May 1;76(5):376-383. PubMed.
Make a Comment
To make a comment you must login or register.