In mTORC and Theta Rhythms, New Clues to How Sleep Locks Down Memories
Quick Links
Parents often insist their children get a good night’s sleep before a big exam, and science says they are onto something. Studies have demonstrated that without sleep, memory traces from the day before vanish. Exactly how sleep strengthens memories, however, remains fuzzy. New studies offer cellular and molecular insights. One reports a protein synthesis pathway essential for memory consolidation, while the second details how consolidation depends on the coordinated firing of neurons in the hippocampus during rapid-eye-movement sleep. Such theta rhythms can falter in people with Alzheimer’s disease. Together the findings offer scientists clues to what might go wrong in people with sleep problems, and may suggest ways to compensate. For more on how sleep helps the brain rid itself of unwanted protein, see Part 1 of this story.
TORC Drives Memories
In the April 26 Science Signaling, researchers led by Ted Abel at the University of Pennsylvania, Philadelphia, pinpointed a complex, mTORC. This kinase complex activates protein translation and is essential for memory consolidation during sleep, the scientists report. Keeping mice awake dampened mTORC activity and the mice flubbed a memory test, whereas artificially stimulating mTORC1 signaling maintained memories even in sleep-deprived animals. “This is the first study to functionally manipulate protein synthesis and reverse the effects of sleep deprivation,” Abel told Alzforum. The findings highlight a crucial role for protein production in memory formation during sleep, he added.

Connecting Sleep and Memory.
Sleep-deprived mice had less mTORC1 signaling, leading to less protein synthesis and a failure to consolidate memories. [Courtesy of Science Signaling/AAAS.]
Commenters called the study a step forward. “The authors have placed our understanding of the molecular neurobiology of sleep deprivation and its deleterious effects on memory cognition on a firm foundation,” wrote David Sweatt and Kimberly Hawkins in an accompanying editorial. Alcino Silva at the University of California, Los Angeles, wrote to Alzforum, “To my knowledge this is the first time that anyone has found a specific molecular pathway, and even better, a specific molecular manipulation, that may help us address the sleep deprivation epidemic that plagues the modern world.” To find such a specific mechanism was surprising, and opens the door to possible interventions to dampen the effects of sleep deprivation, he added. Such interventions might also be applicable to Alzheimer’s and other neurodegenerative diseases in which sleep is often disrupted.
Researchers have long wondered why lack of sleep degrades memory (for review, see Maquet, 2001; Abel et al., 2013). Most research in this area has focused on neurotransmitters and changes in neuronal firing patterns during sleep, but a handful of papers linked protein synthesis during slumber to memory (see Ramm and Smith, 1990; Nakanishi et al., 1997; Seibt et al., 2012). Previously, Abel and colleagues compared hippocampal gene expression in sleep-deprived mice to that in well-rested littermates. They found a marked drop in proteins involved in translation, particularly the mammalian target of rapamycin (mTOR). This cytoplasmic kinase forms part of the insulin signaling pathway and stimulates protein synthesis, although it also subserves cell survival and proliferation (see Vecsey et al., 2012).
Because protein synthesis has been linked to synaptic plasticity, the authors investigated whether it was the process connecting memory impairment to sleep deprivation (see Bourtchouladze et al., 1998; Kelleher et al., 2004; Abraham et al., 2008). First author Jennifer Tudor compared the production of new proteins in the hippocampi of mice that had been deprived of sleep for five hours to that in mice who had just awakened from five hours of slumber. She found a dramatic difference: The sleep-deprived animals had made about half as many new proteins during those five hours as the sleeping ones.
The authors next examined proteins known to interact with mTOR for changes in sleep-deprived mice. They found that lack of sleep kept mTOR from binding Raptor, aka regulatory-associated protein of mTOR, to form the mTORC1 complex. This complex has been studied in depth, and scientists have worked out how it acts in a series of regulated steps that lead to activation of protein synthesis. First, mTORC1 phosphorylates eukaryotic translation initiation factor 4E-binding protein 2 (4EBP2). Phosphorylation of 4EBP2 prevents it from binding to and sequestering eukaryotic initiation factor 4E (eIF4E), and thus frees eIF4E to bind to the related protein eIF4G and turn on translation at the ribosome. In effect, then, sleep releases the brakes on protein translation by activating mTORC1 to phosphorylate 4EBP2 (see image below). Sleep-deprived mice, which did not form the mTORC1 complex, made about half as much phosphorylated 4EBP2 as their rested kin. After they were allowed to sleep, phosphorylated 4EBP2 rose again. Sleep deprivation did not change other proteins known to be involved in this protein synthesis pathway, including the kinase S6K that activates ribosomes, suggesting mTORC1 and 4EBP2 were specifically affected.
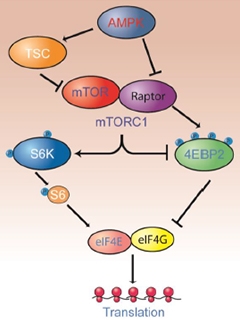
Sleep Toggles Translational Switch.
During sleep, mTORC1 activity inhibits 4EBP2 and activates S6 kinase, releasing the brakes on protein translation in neurons. [Courtesy of Science Signaling/AAAS.]
To find out if this mechanism truly contributed to memory formation during sleep, the authors overexpressed 4EBP2 in hippocampal excitatory neurons using a viral vector. In theory, this overexpression should bump up levels of the phosphorylated version. Three weeks later, mice that received the vector indeed had four times as much phosphorylated 4EBP2 in their hippocampi as did controls—even after sleep deprivation. As predicted, the strategy protected their memories from the effects of lost sleep. The sleepy animals maintained normal amounts of eIF4E bound to eIF4G, and made as much new protein as did rested controls. Moreover, they remembered the locations of objects they had seen before as well as did the rested mice. Sleep-deprived mice injected with a control vector, on the other hand, make less new protein and botched the memory test.
In future work, Abel plans to dissect the regulation of 4EBP2 by looking at specific phosphorylated residues and which phosphatases affect them. He also wants to identify upstream and downstream components of the pathway, by investigating what kicks off signaling through mTORC1 and what proteins are made as a result. In awake animals, insulin triggers this protein-synthesis pathway. Intriguingly, sleep deprivation can lead animals (and people) to eat more, Abel noted. The sugar in food elicits insulin release, thus dialing up protein synthesis and perhaps making up for the lack of it after sleep deprivation. “It would be interesting to see if eating is a compensatory mechanism [for lost sleep],” Abel suggested. In addition, because the same type of memories that are impaired by sleep deprivation also falter in cognitive impairment and dementia, he wondered if similar mechanisms might be at work there.
Sleep Rhythms and Memory
Sleep is not a uniform process. Rather, its stages are distinguished by varying neuronal activity. Are some of these stages more important for memory than others? Researchers led by Sylvain Williams at McGill University, Montreal, and Antoine Adamantidis at the University of Bern, Switzerland, addressed this question in the May 13 Science. They homed in on electrical activity during REM sleep. Low-frequency (i.e., 4-7 Hz) theta rhythms in the brain distinguish REM from other sleep stages, and hippocampal theta rhythms have been linked to memory consolidation (see Poe et al., 2000; Louie and Wilson, 2001). Some studies have correlated disruptions in REM with faulty memory formation, but because REM is difficult to manipulate, researchers could not prove a causal connection (for review, see Stickgold and Walker, 2005). To address this, the authors turned to optogenetics, in which neural circuits are switched on and off with flashes of light (see Nov 2012 series).
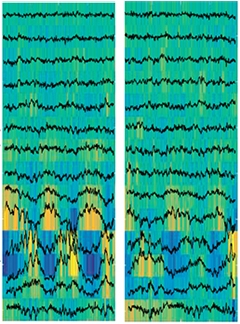
Quieting Theta Rhythms.
Optogenetically silencing GABAergic neurons in the medial septum dampened (right) the theta waves seen normally (left) in the sleeping hippocampus. [Courtesy of Science/AAAS.]
First author Richard Boyce injected a viral vector containing either a photosensitive ion channel or just a fluorescent tag into the medial septum of the brains of transgenic mice. The mice also expressed an activator for the vector, but only in GABAergic neurons of the medial septum. These inhibitory neurons project to the hippocampus and are believed to pace theta rhythms there. The authors then delivered a pulse of light to the medial septum, hyperpolarizing and silencing the neurons. When delivered during REM sleep, this light treatment dropped theta power in the hippocampus by two-thirds. Other parameters of sleep remained normal.
The authors allowed transfected and control mice to explore a cage containing two new objects before the animals bedded down for four hours of sleep. During sleep, researchers optogenetically disrupted REMs in some of the animals. Afterward, they re-introduced them to the two objects, one of which was in a new location. Mice with disrupted REM sleep explored both objects equally, suggesting they did not remember either. Control mice, as well as those that did not receive light stimulation, or only received it during non-REM sleep, remembered their previous play session and mostly investigated the altered toy. The authors obtained similar results using a contextual fear conditioning test: Mice that lacked normal REM sleep failed to remember previous training.
Both of these cognitive tests involve spatial memory. The data suggest a role for REM sleep theta rhythms in locking down hippocampal spatial memories in particular, the authors noted. Intriguingly, some evidence indicates that Alzheimer’s patients have disrupted theta rhythms (see Aug 2010 news). People with AD also have trouble with spatial navigation, frequently becoming lost in their own neighborhoods or even houses (see Mar 2007 news; Oct 2015 news). Whether poor sleep plays a role in this deficit remains to be determined.—Madolyn Bowman Rogers
References
News Citations
- Sleep and Brain Cleansing—Fresh Insights into Regulation and Disruption
- Off Key—Aβ Detunes the Theta Rhythms of the Hippocampus
- “Morris Land Maze” Reveals Early Loss of Spatial Memory in MCI
- Young ApoE4 Carriers Wander Off the ‘Grid’ — Early Predictor of Alzheimer’s?
Series Citations
Paper Citations
- Maquet P. The role of sleep in learning and memory. Science. 2001 Nov 2;294(5544):1048-52. PubMed.
- Ramm P, Smith CT. Rates of cerebral protein synthesis are linked to slow wave sleep in the rat. Physiol Behav. 1990 Nov;48(5):749-53. PubMed.
- Nakanishi H, Sun Y, Nakamura RK, Mori K, Ito M, Suda S, Namba H, Storch FI, Dang TP, Mendelson W, Mishkin M, Kennedy C, Gillin JC, Smith CB, Sokoloff L. Positive correlations between cerebral protein synthesis rates and deep sleep in Macaca mulatta. Eur J Neurosci. 1997 Feb;9(2):271-9. PubMed.
- Seibt J, Dumoulin MC, Aton SJ, Coleman T, Watson A, Naidoo N, Frank MG. Protein synthesis during sleep consolidates cortical plasticity in vivo. Curr Biol. 2012 Apr 24;22(8):676-82. Epub 2012 Mar 1 PubMed.
- Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem. 1998 Sep-Oct;5(4-5):365-74. PubMed.
- Kelleher RJ 3rd, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004 Sep 30;44(1):59-73. PubMed.
- Abraham WC, Williams JM. LTP maintenance and its protein synthesis-dependence. Neurobiol Learn Mem. 2008 Mar;89(3):260-8. Epub 2007 Nov 7 PubMed.
- Poe GR, Nitz DA, McNaughton BL, Barnes CA. Experience-dependent phase-reversal of hippocampal neuron firing during REM sleep. Brain Res. 2000 Feb 7;855(1):176-80. PubMed.
- Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001 Jan;29(1):145-56. PubMed.
- Stickgold R, Walker MP. Sleep and memory: the ongoing debate. Sleep. 2005 Oct;28(10):1225-7. PubMed.
Further Reading
News
- Does Amyloid Disturb the Slow Waves of Slumber—and Memory?
- Sleep Gene Switch Means Lights Out, Memory On, for Fruit Flies
- Remember, Remember…Growth Factor, Timing, and Sleep
- Sleep On It—Astrocytes May Play Key Roles in PD, AD
- Making and Mining Memories—New Insight Into Hippocampal Role
- Smell You Later—Odors Help Consolidate Memory Overnight
- Could Alzheimer’s Drugs Mean “Good Night” to Good Memory?
Primary Papers
- Tudor JC, Davis EJ, Peixoto L, Wimmer ME, van Tilborg E, Park AJ, Poplawski SG, Chung CW, Havekes R, Huang J, Gatti E, Pierre P, Abel T. Sleep deprivation impairs memory by attenuating mTORC1-dependent protein synthesis. Sci Signal. 2016 Apr 26;9(425):ra41. PubMed.
- Sweatt JD, Hawkins KE. The molecular neurobiology of the sleep-deprived, fuzzy brain. Sci Signal. 2016 Apr 26;9(425):fs7. PubMed.
- Boyce R, Glasgow SD, Williams S, Adamantidis A. Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science. 2016 May 13;352(6287):812-6. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
UCLA
I was very impressed with this sleep-mTORC1 study from Ted Abel’s lab. To my knowledge this is the first time that anyone found a specific molecular pathway, and even better, a specific molecular manipulation, that addresses the epidemic of sleep deprivation that plagues the modern world.
This is an enormous problem with far-reaching consequences, many of which we are only starting to realize. Sleep plays a key homeostatic role in most biological processes that is absolutely central to nearly every aspect of our health and well-being. In our busy, overstimulated world, we are all constantly recovering from lack of sleep, whether because of choices we make or because of our jobs and accelerated lives.
Abel’s lab, by demonstrating that 4EBP2-regulated protein synthesis is central to the memory problems caused by loss of sleep, has opened the door to a mechanistic understanding of this serious problem, and to possible interventions to dampen its effects. It is really incredible that the authors found such a specific process: Most of us thought that something as drastic as memory deficits caused by loss of sleep would affect a wide range of molecular, cellular, and circuit processes, and that targeting any one of these would have little or no impact. These findings have changed how I view this problem, and they represent a really important contribution to the field.
Make a Comment
To make a comment you must login or register.