Memory Maker or Neuron Killer? For Transcription Factor, It May Depend on Mood
Quick Links
Could a protein that ramps up in response to stress also hold the key to making memories? According to a paper in the April 9 Cell Reports, the transcription factor ATF4 is necessary for synaptic plasticity in the hippocampus and for spatial memory. These new findings add a twist to previous suggestions that ATF4 scuppers memory, and even helps Aβ do its dirty work. Led by Michael Shelanski at Columbia University in New York City, the study may help clarify the muddy waters surrounding ATF4’s role in neural function.
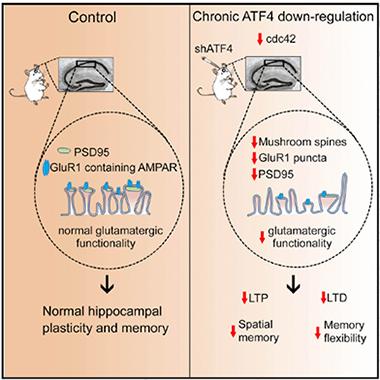
Memory Maker?
Turning down expression of ATF4 in the hippocampus (right panel) reduces mushroom spines, leading to a drop in synaptic plasticity and memory. [Image courtesy of Pasini et al., Cell Reports, 2015.]
ATF4 (Activated Transcription Factor 4) is a member of the CREB family of transcription factors, which are important for formation of long-term memories (see Jan 2010 conference news). In the case of ATF4, the integrated stress response (ISR) regulates its translation. An alarm system rigged to respond to a diverse array of upstream stressors, including protein misfolding and aggregation (see Apr 2015 news), the ISR kicks off with the phosphorylation of elongation initiation factor 2α (eIF2α). Once phosphorylated, eIF2α powers down the translation of most proteins in the cell; a few transcription factors, including ATF4, turn on instead.
The idea that ATF4 plays a negative role in neuronal function emerged in 2003, when Eric Kandel of Columbia University reported that inhibition of ATF4 and related transcription factors led to an improvement in synaptic plasticity and memory in mice (see Chen et al., 2003). Other studies supported the idea, reporting that inhibition of eIF2α phosphorylation, and thus reduction of ATF4 expression, improved memory (see Apr 2007 news on Costa-Mattioli et al., 2007, and Ma et al., 2013). However, a handful of studies drew different conclusions, reporting that eIF2α phosphorylation in the hippocampus was important for certain aspects of memory (see Trinh et al., 2012; ILL-Raga et al., 2013).
Missing from the field was a direct assessment of ATF4’s function, independent of eIF2α phosphorylation and without inhibition of other transcription factors, Shelanski said. Complicating matters, ATF4-null mice are afflicted with a host of problems, including blindness, which makes behavioral tests problematic. First author Silvia Pasini and colleagues decided to take a more targeted approach: They injected short hairpin RNAs that knocked down ATF4 expression into the hippocampuses of adult mice. The group had cut their teeth on this technique in a previous study, in which they reported that loss of ATF4 in the hippocampus reduced the density of dendritic spines (see Liu et al., 2014). In the current study, the researchers wanted to measure whether loss of ATF4 would have real consequences on synaptic plasticity and memory.
One month after knocking down ATF4 expression, the researchers tested the mice on a variety of cognitive tests. They had deficits in long-term spatial memory, as seen by the inordinate amount of time they spent swimming around in the wrong quadrant to find a hidden platform in the Morris water maze test. When the researchers moved the platform to the opposite side of the tank, the mice took longer to get with the new program than mice injected with a control shRNAs. The researchers next tested the mice for associative memory by monitoring their reactions to a sound that had played when they had received a foot shock the day before. All groups of mice performed similarly on this test, suggesting that ATF4 is not required for associative memory. Pasini pointed out that amygdala-based memory could have compensated for a lack of hippocampal memory in this case. The amygdala is the major fear center in the brain.
Did problems with synaptic transmission underlie the behavioral deficits? To find out, the researchers analyzed hippocampal slice cultures for long-term potentiation and long-term depression, two hallmarks of synaptic plasticity. They found that both processes were compromised in cultures from mice that had received ATF4 shRNA. Neurons from 2-month-old ATF4-deficient mice were similarly impaired. Whole cell patch clamp recordings revealed a reduction in both the amplitude and frequency of miniature excitatory postsynaptic currents (mEPSCs) in cultures from ATF4 knockdown mice. Adding back an ATF4 gene that was resistant to shRNA knockdown rescued this deficit. Adding back a mutated version of ATF4 that could not bind DNA failed to restore mEPSC activity, indicating that ATF4’s transcription factor activity is important for synaptic function.
Combining results from this study and their previous one, Pasini and colleagues propose a model in which loss of ATF4 leads to changes in dendritic spine morphology, which reduces synaptic plasticity and learning (see figure above). The researchers are in the process of sifting through bioinformatics data in search of other genes regulated by ATF4 that could be responsible for the deficits.
How does this study jibe with previous ones suggesting that ATF4 weakens memory? Shelanski said most previous studies had assessed ATF4 effects indirectly by inhibiting eIF2α phosphorylation, which has myriad downstream targets. Eric Klann of New York University agreed that other factors regulated by eIF2α could explain some previous results. However, he added that eIF2αand ATF4 could also exert different effects in different settings. For example, Klann’s lab has reported that inhibition of PERK, a kinase that phosphorylates eIF2α, ameliorated deficits in synaptic plasticity and memory in an AD mouse model, yet in another study with normal mice, the same inhibition enhanced memory flexibility (see Aug 2013 news).
Mauro Costa-Mattioli of Baylor College of Medicine in Houston said the paper was interesting, but commented that it will be important to clarify ATF4’s role on memory formation only, avoiding complications such as increased sensitivity to oxidative stress (see Lange et al., 2008). Rather than shutting down ATF4 expression continuously, which may affect secondary functions, researchers could address memory-specific functions by switching off ATF4 only during key behavioral tasks, he said. Costa-Mattioli added that other plasticity-related genes besides ATF4 may also function downstream of eIF2α. He recently reported that eIF2α phosphorylation turns on OPHN1, which is important for long-term depression and learning (see Di Prisco et al., 2014).
Another potential conflict with Shelanski’s findings is the discovery that ISRIB, an inhibitor of the integrated stress response, enhances memory while lowering ATF4 expression (see Sidrauski et al., 2013). However, a new study published April 9 in Science Express reported that the inhibitor works by ramping up the activity of eIF2β. This guanidine exchange factor helps drive initiation of translation by swapping a GDP for a GTP on the initiation complex. It normally switches off when eIF2α is phosphorylated. “Given the findings of Pasini, the memory-enhancing effects of ISRIB are unlikely to reflect impaired ATF4 expression, but rather some other process that is dependent on the integrated stress response,” said senior author David Ron of the University of Cambridge in England.
What about ATF4’s proposed role in Aβ toxicity? A study reported that when it met Aβ oligomers in axons, ATF4 moved back to the cell body, where it initiated cell death (see Aug 2014 news). That study’s senior author, Ulrich Hengst, also at Columbia, said that it is difficult to compare his and Shelanski’s studies directly, due to the different models used and outcomes measured. Hengst also said that ATF4 likely plays different roles depending on context and amount of protein. “With regards to neurodegeneration and AD, it is important to carefully investigate whether there is a threshold below which ATF4 is neuroprotective,” he said.
Shelanski agreed. “We found that ATF4 was necessary to maintain memory, and we view it as maintaining a proper physiological state,” he said. “As with anything, proper balance is key.”—Jessica Shugart
References
News Citations
- Copper Mountain: Can CREB Save Memory?
- Phosphatase Inhibitor Promotes Protein Folding, Helps ALS Mice
- Memories—The Long, the Short, and the Schemas
- Boosting Protein Translation PERKs Up Synapses in Alzheimer's Mice
- Proteins Backpedal, Spreading Neurodegeneration
Paper Citations
- Chen A, Muzzio IA, Malleret G, Bartsch D, Verbitsky M, Pavlidis P, Yonan AL, Vronskaya S, Grody MB, Cepeda I, Gilliam TC, Kandel ER. Inducible enhancement of memory storage and synaptic plasticity in transgenic mice expressing an inhibitor of ATF4 (CREB-2) and C/EBP proteins. Neuron. 2003 Aug 14;39(4):655-69. PubMed.
- Costa-Mattioli M, Gobert D, Stern E, Gamache K, Colina R, Cuello C, Sossin W, Kaufman R, Pelletier J, Rosenblum K, Krnjević K, Lacaille JC, Nader K, Sonenberg N. eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell. 2007 Apr 6;129(1):195-206. PubMed.
- Ma T, Trinh MA, Wexler AJ, Bourbon C, Gatti E, Pierre P, Cavener DR, Klann E. Suppression of eIF2α kinases alleviates Alzheimer's disease-related plasticity and memory deficits. Nat Neurosci. 2013 Sep;16(9):1299-305. PubMed.
- Trinh MA, Kaphzan H, Wek RC, Pierre P, Cavener DR, Klann E. Brain-specific disruption of the eIF2α kinase PERK decreases ATF4 expression and impairs behavioral flexibility. Cell Rep. 2012 Jun 28;1(6):676-88. PubMed.
- ILL-Raga G, Köhler C, Radiske A, Lima RH, Rosen MD, Muñoz FJ, Cammarota M. Consolidation of object recognition memory requires HRI kinase-dependent phosphorylation of eIF2α in the hippocampus. Hippocampus. 2013 Jun;23(6):431-6. Epub 2013 Mar 18 PubMed.
- Liu J, Pasini S, Shelanski ML, Greene LA. Activating transcription factor 4 (ATF4) modulates post-synaptic development and dendritic spine morphology. Front Cell Neurosci. 2014;8:177. Epub 2014 Jun 30 PubMed.
- Lange PS, Chavez JC, Pinto JT, Coppola G, Sun CW, Townes TM, Geschwind DH, Ratan RR. ATF4 is an oxidative stress-inducible, prodeath transcription factor in neurons in vitro and in vivo. J Exp Med. 2008 May 12;205(5):1227-42. Epub 2008 May 5 PubMed.
- Di Prisco GV, Huang W, Buffington SA, Hsu CC, Bonnen PE, Placzek AN, Sidrauski C, Krnjević K, Kaufman RJ, Walter P, Costa-Mattioli M. Translational control of mGluR-dependent long-term depression and object-place learning by eIF2α. Nat Neurosci. 2014 Aug;17(8):1073-82. Epub 2014 Jun 29 PubMed.
- Sidrauski C, Acosta-Alvear D, Khoutorsky A, Vedantham P, Hearn BR, Li H, Gamache K, Gallagher CM, Ang KK, Wilson C, Okreglak V, Ashkenazi A, Hann B, Nader K, Arkin MR, Renslo AR, Sonenberg N, Walter P. Pharmacological brake-release of mRNA translation enhances cognitive memory. Elife. 2013;2:e00498. PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Pasini S, Corona C, Liu J, Greene LA, Shelanski ML. Specific downregulation of hippocampal ATF4 reveals a necessary role in synaptic plasticity and memory. Cell Rep. 2015 Apr 14;11(2):183-91. PubMed.
- Sekine Y, Zyryanova A, Crespillo-Casado A, Fischer PM, Harding HP, Ron D. Stress responses. Mutations in a translation initiation factor identify the target of a memory-enhancing compound. Science. 2015 May 29;348(6238):1027-30. Epub 2015 Apr 9 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.