Long Live the Microglia! Studies Trace Their Lifespans in Mice and Humans
Quick Links
At the ripe age of 15 months, Hildegard died. Fortunately, the sting of her passing was relieved by the arrival of her neighbor’s new daughter, Agatha. Before you reach for that box of tissues, know that these are mouse microglia we are naming. According to a study published in Nature Neuroscience on August 28, Hildegard was unlucky, because half of the microglia live for the mouse’s entire lifespan. Researchers led by Mathias Jucker of the University of Tübingen and the German Center for Neurodegenerative Diseases made this discovery by tracking individual cells in mice for more than a year—long enough to consider naming them, said Jucker. The researchers found that in wild-type mice, microglia died at a rate of about 26 percent per year, and an equal percentage of new ones formed. In an AD mouse model, however, microglia distant from plaques proliferated at triple the rate of their loss, and the new cells appeared to migrate toward the plaques. The findings suggest that microglia maintain a slow, balanced turnover when skies are calm, but kick into high proliferative gear when neurodegenerative storm clouds gather.
In another study published in Cell Reports on July 25, researchers led by Jonas Frisén of the Karolinska Institute in Stockholm leveraged traces of carbon-14 left over from atomic bombs to estimate the lifespan of microglia in the human brain. They reported that human microglia live for an average 4.2 years, and that nearly a third of the cells are replenished annually.
“Both of these papers are extremely important for the microglial field,” commented Oleg Butovsky of Brigham and Women’s Hospital in Boston. “The question of microglial turnover is a fundamental one.”
As the macrophages of the brain, microglia are tasked with responding to emerging threats. They migrate to the brain from the embryonic yolk sac before birth (Feb 2015 news). From their posts in the brain, microglia can be “primed” by events happening throughout the body, including shifts in the gut microbiome (Jun 2015 news). How, or even if, these life events influence the way microglia later respond to neurodegenerative diseases depends markedly on how long the cells live.
Multiple studies have attempted to answer this beguilingly simple question, but results conflict. Some data paint microglia as rapidly renewing, while others suggest these immune cells are more like neurons, persisting for the lifespan of their hosts (Jan 2017 news on Askew et al., 2017; Jun 2017 news on Tay et al., 2017).
First author Petra Füger and colleagues approached the question with a combination of live two-photon imaging and a commitment to monitor cells for the long haul. They generated transgenic mice in which only a small proportion of CD11b-positive cells were labeled with the fluorescent protein tdTomato; all Iba1-positive microglia expressed green fluorescent protein. They insured that only 2 percent of CD11b-positive cells expressed tdTomato by dosing the mice once with a small amount of tamoxifen. This triggered a recombination event that turned on the tdTomato gene. Then the researchers selected four to six regions of the neocortex, each containing at least one tdTomato-labeled microglial cell, to image through implanted cranial windows every two weeks for six months, and every month thereafter. Each mouse skull was fitted with a custom titanium ring, which allowed researchers to reattach the microscope to exactly the same place each time with micrometer precision, allowing them to re-image the same cells repeatedly. The animals roamed freely in their cages between imaging sessions. The researchers conducted this intensive monitoring on 27 mice starting at four months of age, and on six mice starting at 10 months of age, for as long as 15.5 months.
Remarkably, the researchers found that the vast majority of microglia were still alive even after imaging for 15 months. However, some microglia did vanish between sessions. Because the researchers chose imaging volumes larger than a microglial cell can travel, they concluded that missing microglial cells had died, rather than migrated away. Death rates in older mice were slightly higher than in younger ones. Because most of the cells were still alive by the last imaging session, the researchers could only conclude with certainty that their lifespan was at least 15 months. However, using the death rate to extrapolate further, the researchers estimated that roughly half of the microglia would persist throughout the 26- to 28-month lifespan of the mice.
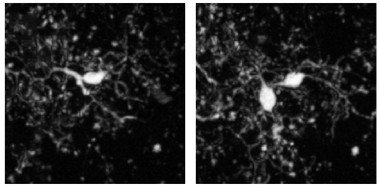
… And Then There Were Two.
New microglia appeared (right) next to existing ones (left). The new cells always persisted in subsequent imaging sessions. [Courtesy of Füger et al., Nature Neuroscience, 2017.]
New microglia also appeared throughout the study. In the four-month-old mice, the researchers calculated that an equal percentage—about 13 percent—of cells appeared and disappeared over the ensuing six months. This is in keeping with the stable numbers of neocortical microglia observed in the mouse brain throughout life (Hefendehl et al., 2014). Interestingly, new cells always appeared in close proximity to existing ones, suggesting that they were a product of cell division. After division, one of the two cells would then migrate, positioning itself about 40 μm away.
Life and death were less balanced when CD11b-tdTomato/Iba1-GFP double transgenic mice were crossed to an APP/PS1 background. These animals develop Aβ plaques by 1.5 months of age. By four months of age, the researchers found that microglia that were not directly associated with plaques disappeared at a rate of about 40 percent per year, faster than in non-AD mice. Strikingly, these non-plaque-associated microglia proliferated three times as fast as they died. And, rather than squirming 40 μm in any direction, these new cells moved toward plaques. Once there, they appeared to further divide and die at approximately equal rates of about 50 percent over six months, though Jucker pointed out that this percentage is not entirely accurate because the cells migrated toward plaques at different times throughout the study, complicating the analysis.
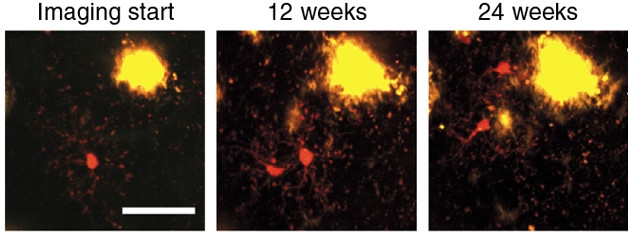
Divide and Conquer? In APP/PS1 mice, microglia (red) divide while away from plaques, then migrate toward the action. [Courtesy of Füger et al., Nature Neuroscience 2017.]
Microglia are known to proliferate in response to threats, including Aβ plaques (Olmos-Alonso et al., 2016). Jucker told Alzforum he was surprised to find the cells did so before coming into contact with plaques. This suggests that something distal from plaques triggers microglia to divide.
Jucker emphasized that it was impossible to tell whether the cell division was symmetric, producing two equal cells, or asymmetric, producing microglial offspring with different qualities than their parents. Christian Haass of the German Center for Neurodegenerative Diseases in Munich agreed that the fate of these cells was critical to address, as well as how the function of these long-lived immune cells change with age. “All these questions will have an important influence on the current search for microglial modulating therapeutic strategies,” he wrote to Alzforum (see full comment below). Jucker said that the researchers in his lab are investigating the nature of the cell division, as well as hunting for the signals that trigger it.
Transcriptional profiling would be one way to assess differences between plaque-associated and non-associated microglia, commented Butovsky. He also wondered whether the small percentage of tdTomato-labeled microglia in Jucker’s mice were representative of the broader microglial population, a question future studies could also answer through profiling. He added that Jucker’s finding that the tracked microglia proliferate in AD mice further supports the idea that plaque responders are brain-resident microglia, not infiltrating monocytes. Whether these cells are outsiders or insiders has been debated for more than a decade (Feb 2006 news; May 2016 news).
Terrence Town of the University of Southern California in Los Angeles called Jucker’s paper a technical tour de force. That said, he pointed out that CD11b and Iba1—the two markers the researchers depended on to track the cells—are also present on brain-infiltrating peripheral monocytes, making it impossible to formally rule out the recruitment of these circulating cells to plaques. The authors acknowledged this possibility in the paper as well. Town was intrigued by the apparent proliferation of microglia away from plaques, adding that whether those cells ultimately gathered around plaques was an important question to answer. That microglia are long-lived in the mouse brain provides a possible explanation for why the cells appear unable to clear plaques, he added. “They’ve spent their entire lives in a relatively immune-privileged environment, so they may lack the capacity to mount an effective response,” Town said.
Diego Gomez-Nicola of the University of Southampton in the U.K., who had previously reported that microglia turn over rapidly, echoed Butovsky’s concerns about selective labeling of microglial cells, wondering if some microglial subtypes might be more prone to the tamoxifen-induced recombination used to turn on tdTomato (Askew et al., 2017). He added that rapid cell division or death events could have contributed to the contradictory findings. “By imaging the same cells every two weeks, it is possible that several cycles of proliferation-death are missed, therefore leading to an underestimation of microglial turnover,” he said (see full comment below).
Jucker and colleagues acknowledged that it was impossible to completely rule out the contribution of missed microglial turnover in between biweekly imaging sessions. However, in a subset of mice that they imaged every day for 10 days, they found very low rates of turnover, making significant replacement within those two-week windows unlikely, they concluded.
Jucker’s finding that microglia are long-lived meshes with recent work led by Marco Prinz of the University of Freiburg in Germany, which employed a different transgenic method to compare microglial turnover across regions of the brain (Tay et al., 2017). “The long-term tracking of neocortical microglia … reproduces our findings that cortical microglia are more likely to survive the entire lifespan of a mouse than microglia in other brain regions,” commented that study’s first author, Tuan Leng Tay of Freiburg. “In contrast to the cortex, our lineage analysis also predicted more rapid turnover of microglia in the hippocampus and cerebellum, which correlates to higher immune surveillance and bioenergetics reported for microglia residing in these compartments,” Tay added.
How does the longevity of microglia in the human brain measure up to that in mice? First author Pedro Réu and colleagues addressed this question in the Cell Reports paper. Because peering through cranial windows at fluorescent cells is not an option in living people, the researchers turned to forensic techniques. The flurry of atomic bomb tests in the 1950s and '60s doubled the amount of radioactive carbon-14 in the atmosphere, which has been steadily decaying ever since. Because DNA incorporates this so-called “bomb pulse” carbon, researchers can use it to estimate the age of a cell—a technique Frisén developed more than a decade ago to demonstrate the longevity of neurons (Jul 2005 news). In the current study, the researchers used flow cytometry to isolate microglial cells from fresh postmortem cortical samples derived from 12 donors born over a span of six decades, and measured the amount of carbon-14 in their DNA.

Atomic Clock.
Using a radioactive signature from atomic bomb tests, researchers estimated that nearly a third of microglia in the human brain turn over each year. [Courtesy of Réu et al., Cell Reports, 2017.]
They calculated that on average, microglia were 4.2 years old, although they ranged from newborn cells to cells that had not divided for more than 20 years. An estimated 28 percent of microglia replenished each year. The researchers also estimated turnover of other cell types in the brain, finding that microglial cells had a renewal rate in between that of rapidly proliferating immune cells, such as granulocytes and monocytes, and that of slowly or non-proliferating neurons.
Butovsky noted that unlike transgenic methods in mice, which come with the caveat of selective labeling of certain microglial populations, carbon-14 integrates into all DNA equally. “That’s the beauty of this work,” he said. “They’ve developed a truly unbiased technique to look at microglial turnover in the human brain.”
Relative to the lifespan of their hosts, microglial turnover in humans appeared to be far more frequent than what Jucker observed in mice. Why the difference? Jucker speculated that differences in environment could explain it: The mice lived in clean, pathogen-free conditions, while humans encounter countless insults that may rouse microglia throughout life. Recent studies have also revealed fundamental differences between human and mouse microglia at the gene expression level (Jun 2017 news).
Jucker added that despite these differences, it is of paramount importance to understand the life cycle of murine microglia. “After all, this is the system in which most of us do our experiments,” he said.
Gomez-Nicola pointed out that the estimated 4.2-year lifespan of human microglia means the cells likely undergo multiple renewal cycles in their lifetime. “An exciting follow-up will be to understand how a constant remodeling of the microglial population affects the physiology of their surrounding cells, as in, for example, microglia’s recently discovered influence on synaptic activity,” he wrote.
Despite the higher number of microglial renewal cycles in the human than the mouse lifespan, microglia are remarkably long-lived relative to other immune cells, according to Ari Waisman of the University of Mainz, Germany. “The implications of [Frisén’s] study is that unlike other hematopoietic cells, microglia cells are ‘old.’ They originate from cells that reached the CNS in the embryonic stage, and their progenitors are not fresh cells that come via the blood to the CNS,” Waisman wrote to Alzforum. “This means that the microglia age just like other cells of the CNS.”—Jessica Shugart
References
News Citations
- Nature Versus Nurture: What Gives Microglia Their Identity?
- To Be Hale and Hearty, Brain Microglia Need a Healthy Gut
- Aging Causes “Identity Crisis” in Glia
- Hot DAM: Specific Microglia Engulf Plaques
- Calling for Backup: Microglia from Bone Marrow Fight Plaques in AD Mice
- Barrier Function: TREM2 Helps Microglia to Compact Amyloid Plaques
- Dating the Birth of Human Cells—Carbon 14 Runs Rings around Competition
- Human and Mouse Microglia Look Alike, but Age Differently
Research Models Citations
Paper Citations
- Askew K, Li K, Olmos-Alonso A, Garcia-Moreno F, Liang Y, Richardson P, Tipton T, Chapman MA, Riecken K, Beccari S, Sierra A, Molnár Z, Cragg MS, Garaschuk O, Perry VH, Gomez-Nicola D. Coupled Proliferation and Apoptosis Maintain the Rapid Turnover of Microglia in the Adult Brain. Cell Rep. 2017 Jan 10;18(2):391-405. PubMed.
- Tay TL, Mai D, Dautzenberg J, Fernández-Klett F, Lin G, Sagar, Datta M, Drougard A, Stempfl T, Ardura-Fabregat A, Staszewski O, Margineanu A, Sporbert A, Steinmetz LM, Pospisilik JA, Jung S, Priller J, Grün D, Ronneberger O, Prinz M. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat Neurosci. 2017 Jun;20(6):793-803. Epub 2017 Apr 17 PubMed.
- Hefendehl JK, Neher JJ, Sühs RB, Kohsaka S, Skodras A, Jucker M. Homeostatic and injury-induced microglia behavior in the aging brain. Aging Cell. 2014 Feb;13(1):60-9. Epub 2013 Sep 18 PubMed.
- Olmos-Alonso A, Schetters ST, Sri S, Askew K, Mancuso R, Vargas-Caballero M, Holscher C, Perry VH, Gomez-Nicola D. Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer's-like pathology. Brain. 2016 Mar;139(Pt 3):891-907. Epub 2016 Jan 8 PubMed.
Further Reading
No Available Further Reading
Primary Papers
- Réu P, Khosravi A, Bernard S, Mold JE, Salehpour M, Alkass K, Perl S, Tisdale J, Possnert G, Druid H, Frisén J. The Lifespan and Turnover of Microglia in the Human Brain. Cell Rep. 2017 Jul 25;20(4):779-784. PubMed.
- Füger P, Hefendehl JK, Veeraraghavalu K, Wendeln AC, Schlosser C, Obermüller U, Wegenast-Braun BM, Neher JJ, Martus P, Kohsaka S, Thunemann M, Feil R, Sisodia SS, Skodras A, Jucker M. Microglia turnover with aging and in an Alzheimer's model via long-term in vivo single-cell imaging. Nat Neurosci. 2017 Oct;20(10):1371-1376. Epub 2017 Aug 28 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
University of Southampton
Füger et al. use an inheritable tagging system to track individual microglia over prolonged periods of time. By imaging the same cells every two weeks, it is possible that several cycles of proliferation/death are missed, therefore leading to an underestimation of microglial turnover. This could explain the discrepancy of their findings with those reported by us and others, including Réu and colleagues, wherein the turnover rates suggested the microglial population renews several times in a lifetime (Babcock et al., 2013; Askew et al., 2017; Tay et al., 2017). It is also unclear how representative the tagged population is when compared to all microglia, as their sensitivity to tamoxifen-induced recombination could suggest a different phenotype. It will be useful to see similar studies replicated in the future, by using alternative methodological approaches.
Füger et al.’s study of microglial turnover in a mouse model of AD-like pathology provides a useful replication of previously reported findings by our group and others (Olmos-Alonso et al., 2016) and puts microglial proliferation in the spotlight of AD pathology.
In this sense, the study by Réu et al. is very useful and provides a human “reality check” to compare murine studies against. Although some method-dependent variability was documented in this paper, the authors provide solid evidence that the degree of microglial turnover in people is higher than thought before. This is of particular importance for humans, as our longer life expectancy makes the microglial population susceptible to multiple renewal cycles in a lifetime.
An exciting follow-up of these and previous studies will be to understand how a constant remodeling of the microglial population impacts on the physiology of their surrounding cells, as in, for example, microglia’s recently discovered influence on synaptic activity.
References:
Babcock AA, Wirenfeldt M, Finsen B. Quantification of microglial proliferation and apoptosis by flow cytometry. Methods Mol Biol. 2013;1041:129-45. PubMed.
Askew K, Li K, Olmos-Alonso A, Garcia-Moreno F, Liang Y, Richardson P, Tipton T, Chapman MA, Riecken K, Beccari S, Sierra A, Molnár Z, Cragg MS, Garaschuk O, Perry VH, Gomez-Nicola D. Coupled Proliferation and Apoptosis Maintain the Rapid Turnover of Microglia in the Adult Brain. Cell Rep. 2017 Jan 10;18(2):391-405. PubMed.
Tay TL, Mai D, Dautzenberg J, Fernández-Klett F, Lin G, Sagar, Datta M, Drougard A, Stempfl T, Ardura-Fabregat A, Staszewski O, Margineanu A, Sporbert A, Steinmetz LM, Pospisilik JA, Jung S, Priller J, Grün D, Ronneberger O, Prinz M. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat Neurosci. 2017 Jun;20(6):793-803. Epub 2017 Apr 17 PubMed.
Olmos-Alonso A, Schetters ST, Sri S, Askew K, Mancuso R, Vargas-Caballero M, Holscher C, Perry VH, Gomez-Nicola D. Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer's-like pathology. Brain. 2016 Mar;139(Pt 3):891-907. Epub 2016 Jan 8 PubMed.
University of Freiburg
It is excellent to see that Füger et al. have succeeded with longitudinal live imaging of steady-state microglial network over a reasonably long period and with such good resolution. We attempted to do this with the Microfetti mouse (Tay et al., 2017), but the XFP signals were not sufficiently bright for standard two-photon microscopy to perform the analysis reliably. The long-term tracking of neocortical microglia in this new study reproduces our predictions that cortical microglia are more likely to survive the entire lifespan of a mouse than microglia in other brain regions. In contrast to the cortex, our lineage analyses also pointed to more rapid turnover of microglia in the hippocampus and cerebellum, which correlates to higher immune surveillance and bioenergetics reported for microglia residing in these compartments (Grabert et al., 2016). The observation that non-plaque-associated microglia proliferated threefold quicker in the AD mouse model than in wildtype mouse, coupled with Reu et al.’s evidence that about a third of human microglia turn over in a year, raises the question of whether high turnover is a sign of microglial exhaustion, or if it increases the susceptibility for disease. Or both.
I am excited about how quickly the field is moving and that many groups are making significant contributions to the progress. In particular, I wonder how we can now reconcile the properties of the disease-associated microglia (DAM) published recently (Keren-Shaul et al., 2017) with the hypothesis of microglial recruitment to plaques proposed in the Füger study. Are these the same type of microglial cells? Does a subset of the non-plaque-associated microglia exclusively adopt a DAM signature and therefore move toward a plaque? If so, would this be a chance event, or are highly proliferative microglial cells more prone to becoming DAM? Would it be informative to perform long-term imaging of microglia of AD mouse models in a Trem2-/- or Tyrobp -/- background? Also, how do we interpret this hypothesis in relation to the data showing BrdU-labeled microglia surrounding a plaque, albeit it is not clear if the authors distinguished between plaque- and non-plaque-associated microglia in this study (Olmos-Alonso et al., 2016)?
References:
Tay TL, Mai D, Dautzenberg J, Fernández-Klett F, Lin G, Sagar, Datta M, Drougard A, Stempfl T, Ardura-Fabregat A, Staszewski O, Margineanu A, Sporbert A, Steinmetz LM, Pospisilik JA, Jung S, Priller J, Grün D, Ronneberger O, Prinz M. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat Neurosci. 2017 Jun;20(6):793-803. Epub 2017 Apr 17 PubMed.
Grabert K, Michoel T, Karavolos MH, Clohisey S, Baillie JK, Stevens MP, Freeman TC, Summers KM, McColl BW. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat Neurosci. 2016 Mar;19(3):504-16. Epub 2016 Jan 18 PubMed.
Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I. A Unique Microglia Type Associated with Restricting Development of Alzheimer's Disease. Cell. 2017 Jun 15;169(7):1276-1290.e17. Epub 2017 Jun 8 PubMed.
Olmos-Alonso A, Schetters ST, Sri S, Askew K, Mancuso R, Vargas-Caballero M, Holscher C, Perry VH, Gomez-Nicola D. Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer's-like pathology. Brain. 2016 Mar;139(Pt 3):891-907. Epub 2016 Jan 8 PubMed.
Biomedizinisches Centrum (BMC), Biochemie & Deutsches Zentrum für Neurodegenerative Erkrankungen (DZNE)
Although there were several attempts to determine the age and turnover of microglia in the past, this is what the field has been waiting for for a long time! Now, we know it: Mice are basically born not only with most, if not all, of their neurons and oligodendrocytes, but also with most of their microglia. At least in mice, microglia seem to survive through an entire life span! Of course, we must keep in mind that laboratory mice are kept under extremely artificial, super-clean conditions, which certainly will influence microglia survival tremendously and almost certainly reduce heterogeneity of microglial populations (see below).
And, are we aging with an increasing set of "primed" microglia? Does this finding mean we may have long-lived populations of all microglia that have been primed for all types of pathological insults that once happened in our brain? And what happens when these microglia age? Do they lose their function, e.g., to fight neuropathology during the progression of AD?
The Jucker lab also showed that microglia proliferate in response to disease pathology. This finding leads to the important question of the fate of these cells. Do old cells divide "asymmetrically” and produce young "offspring”? All these questions will have an important influence on the current search for microglial modulating therapeutic strategies.
Yes, we must keep in mind that in humans the situation may be slightly different, probably because we are challenged constantly by all types of pathological insults. The paper by Reu et al. shows that very nicely. They determined the lifespan and turnover of human microglia by the analysis of the integration of atmospheric 14C derived from nuclear bomb tests into genomic DNA. Their results in humans show some important differences to mice: Human microglia have an average age of 4.2 years and about 28 percent are replaced every year. Thus, most human microglia are indeed renewed throughout life and there is no evidence for very long-lived microglia as in mice. The questions, however, are the same. What is the state of the renewed microglia? Are they fully functional in a homeostatic state and fully capable of quickly responding to pathological insults?
Microglia are macrophages of the central nervous system (CNS) and as such belong the hematopoietic system that also includes, for example, B and T cells. What is special for microglia cells is that they seed the CNS very early during embryonic development and probably very little after birth. In other words, most evidence so far, almost all from studies with mice, show that these cells are maintained in the CNS through adulthood, but do not need a constant feed from the bone marrow, unlike other cells of the hematopoietic origin.
This from Réu et al. reports interesting findings and provides a strong argument on the turnover rate of human microglia cells. The authors have shown that in humans, and not only in mice, microglia cells are renewed slowly from long-lived cells that are present in the CNS. The implications of this study are that unlike other hematopoietic cells, microglia are “old.” They originate from cells that reached the CNS in the embryonic stage and their progenitors are not fresh cells that come via the blood to the CNS. This means that the microglia age just like other cells of the CNS.
Make a Comment
To make a comment you must login or register.