Human Tau Strains Propagate Faithfully in Wild-Type Mice
Quick Links
In the October 20 Journal of Neuroscience, researchers led by Virginia Lee at the University of Pennsylvania, Philadelphia, added evidence to the idea that the particular conformation of misfolded proteins dictates how fast pathology spreads through the brain and what cell types it affects. The researchers isolated tau fibrils from the brains of people who had had Alzheimer’s, corticobasal degeneration (CBD), or progressive supranuclear palsy (PSP), and injected them into the brains of wild-type mice. There, the isolates seeded pathology reminiscent of the human disease they came from, with CBD and PSP tau spreading faster and affecting glial cells, while AD tau remained neuronal. “This shows you can model different neurodegenerative tauopathies in wild-type mice. These models will help us understand mechanisms of spreading and identify therapies,” Lee told Alzforum.
- Tau fibrils from human AD, PSP, and CBD cases seed pathology in wild-type mice.
- Each aggregate triggers pathology reminiscent of the human disease from which it came.
- The results support the existence of specific strains of toxic protein.
“It’s impressive that the human tauopathies replicate so well in the mouse model,” agreed Lary Walker at Emory University, Atlanta. “One of the big challenges in the field is to determine the mechanisms of cell-type specificity in neurodegenerative disease. This model provides a framework in which to address that question.”
Researchers had previously found that aggregated tau could seed pathology in the brains of young transgenic mice (Jun 2009 news). Last year, Lee and colleagues reported that purified tau fibrils from AD brains, but not synthetic tau aggregates, triggered tau deposition in wild-type mice as well (Guo et al., 2016).
First author Sneha Narasimhan extended this work to PSP and CBD, two pure tauopathies that progress rapidly and rob people of movement and aspects of cognition. The authors took samples from the frontal cortices of postmortem brains with AD, PSP, or CBD, and purified the resulting lysate to enrich for insoluble tau deposits. Without this enrichment, the lysates were too inert to trigger tau deposition, Lee noted. Even after enrichment, tau protein made up only about 1 or 2 percent of the PSP and CBD lysates. Tau deposits are relatively sparse in these diseases compared to AD, Lee said. Lysates from each disease varied in how stable they were and what bands formed after Proteinase K digestion, suggesting they consisted of different protein strains.
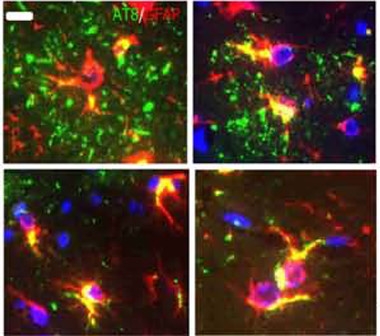
Distinct Strains?
Tau aggregates (green) from CBD brains (top panels) form plaques in astrocytes (red; overlay appears yellow). Tau from PSP brain (bottom) appears in “tufted” astrocytes characteristic of that disease. [Courtesy of Narasimhan et al., The Journal of Neuroscience 2017.]
The authors injected these samples into the hippocampi of wild-type mice, and analyzed the results three months later. All the lysates seeded mouse tau pathology, but with strikingly different results. The AD tau produced the fewest deposits, while CBD tau was more potent, spreading farther through the brain. PSP tau spread the fastest. In particular, tau extracted from two PSP patients who had extensive frontal cortical tau pathology triggered the most aggressive disease, suggesting these cases harbored a particularly potent strain.
The strains also had unique effects on each cell type. AD tau seeded aggregation only in neurons, and mostly hilar neurons at that. CBD tau and PSP tau affected additional neuronal subtypes and also triggered tau deposition in oligodendrocytes and astrocytes. In the latter, CBD tau deposits resembled the astrocytic plaques seen in the human disease, while PSP tau frequently caused “tufted” astrocytes like those in human PSP (see image above). “The most interesting part is that you can demonstrate strain specificity in the wild-type mouse that recapitulates what you see in human brain,” Lee told Alzforum.
Pathology appeared to spread along anatomical brain connections. In the case of oligodendrocytes, deposits appeared along white-matter tracts, suggesting aggregates might be passed directly between these cells. For astrocytes, extensive tau deposits in a particular brain region were linked with few neuronal deposits there, suggesting neurons might be passing off aggregates to astrocytes. To test this idea, Lee plans to inject tau aggregates into wild-type mice that lack neuronal tau, and see if glia alone are able to propagate the pathology.
One limitation of this study was the impurity of its preparations. Fibrils have been shown previously to comprise multiple protein strains (Sanders et al., 2014). Lee told Alzforum she has been unable to serially propagate these preparations in vitro, another requirement for defining specific strains. To obtain purer strains, Lee is attempting to expand the lysate material in vitro using recombinant protein. If she can generate homogenous strains from recombinant tau and still seed tau aggregation, then structural analysis of the strains could provide insight into why they behave as they do in the brain, she noted.—Madolyn Bowman Rogers
References
News Citations
Paper Citations
- Guo JL, Narasimhan S, Changolkar L, He Z, Stieber A, Zhang B, Gathagan RJ, Iba M, McBride JD, Trojanowski JQ, Lee VM. Unique pathological tau conformers from Alzheimer's brains transmit tau pathology in nontransgenic mice. J Exp Med. 2016 Nov 14;213(12):2635-2654. Epub 2016 Oct 17 PubMed.
- Sanders DW, Kaufman SK, DeVos SL, Sharma AM, Mirbaha H, Li A, Barker SJ, Foley AC, Thorpe JR, Serpell LC, Miller TM, Grinberg LT, Seeley WW, Diamond MI. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron. 2014 Jun 18;82(6):1271-88. Epub 2014 May 22 PubMed.
Further Reading
Primary Papers
- Narasimhan S, Guo JL, Changolkar L, Stieber A, McBride JD, Silva LV, He Z, Zhang B, Gathagan RJ, Trojanowski JQ, Lee VM. Pathological Tau Strains from Human Brains Recapitulate the Diversity of Tauopathies in Nontransgenic Mouse Brain. J Neurosci. 2017 Nov 22;37(47):11406-11423. Epub 2017 Oct 20 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
RIKEN Center for Brain Science
The results are very interesting. I wonder if there are specific substances, such as proteins and lipids bound to the pathogenic tau, that promote propagation.
Make a Comment
To make a comment you must login or register.