FTLD Gene Bad Actor in Many TDP-43 Proteinopathies
Quick Links
A variant of the TMEM106B gene, originally discovered in people with frontotemporal lobar degeneration with TDP-43 pathology, influences the TDP-43 protein in other neurodegenerative diseases too, according to a study in the February 4 Neurology online. Researchers at the Rush Alzheimer’s Disease Center in Chicago detected the variant in people with TDP-43 pathology but without FTLD, including those who were cognitively normal or had Alzheimer’s. Senior author Julie Schneider and colleagues also found that the polymorphism affected the expression of TMEM106B itself and of the FTLD gene progranulin, but the scientists still need to work out how those and other genes control TDP-43 pathology.
TDP-43 forms abnormal cytoplasmic inclusions in the brains of people with various neurodegenerative diseases, including amyotrophic lateral sclerosis, FTLD, as well as a fraction of cases of Alzheimer’s, Lewy body disease, and hippocampal sclerosis. TDP-43 was once viewed as a marker of damaged neurons; however, lately it has been shown to follow a standard pathway throughout the brain and correlate with clinical symptoms, write Keith Josephs of the Mayo Clinic in Rochester, Minnesota, and Peter Nelson of the University of Kentucky in Lexington, in an editorial accompanying the Neurology article (see Nov 2013 news; Jan 2014 news). “TDP-43 should join the inner circle of neurodegenerative misfolded proteins that now include β-amyloid, tau, and α-synuclein,” they propose.
What leads this newly appreciated pathological protein to aggregate? In 2010, a genome-wide association study offered up a hint: A stretch of DNA on chromosome seven, containing the gene TMEM106B, was associated with FTLD with TDP-43 pathology (see Feb 2010 news). Little was known about this gene, which encodes a transmembrane protein. Since then, researchers have determined that TMEM106B controls lysosome trafficking (see Aug 2012 news; Swenk et al., 2014; Stagi et al., 2014).
The original GWAS finding was based on an adenine/guanine polymorphism at a non-coding site near TMEM106B. The guanine indicated a 60 percent lower risk for FTLD. Researchers have not yet determined the sequence responsible for that protection, but they believe it must be a different variant that co-inherits with the guanine. In vitro studies suggest it might be a threonine-to-serine substitution at amino acid 185 of TMEM106B (Nicholson et al., 2013).
Going Beyond FTLD
Yu and colleagues wanted to understand how TMEM106B contributes to the spread of pathological TDP-43 across the brain in diseases other than FTLD. They analyzed autopsied brain tissue of 544 people, aged 71 or older at time of death, who did not meet pathological criteria for FTLD (see Cairns et al., 2007). As is typically the case with non-demented old people, the majority—82 percent—did have some sort of brain pathology, including plaques and tangles, Lewy bodies, hippocampal sclerosis, or a combination thereof. Before they died, about 70 percent of tissue donors across these pathologies had signs of cognitive impairment, Yu told Alzforum. Half of the brains had some level of TDP-43 pathology, and the higher-risk adenine allele associated with more advanced stages.
TMEM106B influences TDP-43 proteinopathy “above and beyond FTLD,” concluded Yu. Other researchers have also linked the TMEM106B to Alzheimer’s disease, hippocampal sclerosis, Lewy body disease, and amyotrophic lateral sclerosis (Rutherford et al., 2012; Nelson et al., 2015; Aoki et al., 2015; Vass et al., 2011).
Moreover, the correlation between TMEM106B status and TDP-43 spread held true regardless of any other neuropathology. “Even in normal people, TMEM106B genotype influences propensity to form TDP-43 pathology,” commented Alice Chen-Plotkin of the University of Pennsylvania School of Medicine, who was not involved in the study. She suggested those healthy individuals with the TMEM106B adenine allele and TDP-43 pathology may have taken one step on the road to FTLD or other neurodegenerative disease. Subsequent hits might be required to reach a symptomatic state, she speculated.
“It suggests that neurodegeneration could be a really long, slow process,” added Chen-Plotkin. As with the amyloid of Alzheimer’s and the α-synuclein of Parkinson’s, which take root well before thinking or movement are altered, TDP-43 seems to infiltrate the brain before a person shows signs of FTLD or other disease, she said.
TMEM106B and Gene Expression
Having shown that TMEM106B genotype affected TDP-43 proteinopathy, Yu and colleagues next analyzed how that might occur (see image below). TMEM106B has been shown to regulate its own expression as well as that of progranulin, another FTLD gene (see Nov 2010 news; Finch et al., 2011). Yu measured RNA amounts in the autopsy tissues, and confirmed that both TMEM106B and PGRN were upregulated in the people with the high-risk adenine TMEM106B allele.
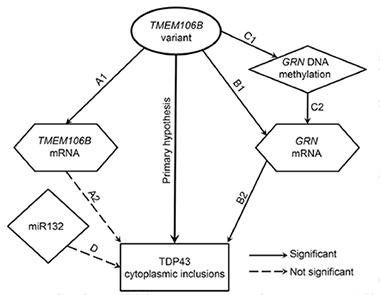
Potential Pathways.
Several hypotheses for how TMEM106B genotype influences TDP-43 pathology. [Republished with permission, © 2015 American Academy of Neurology.]
Because other scientists have hypothesized that methylation suppresses progranulin expression, Yu and Schneider checked the status of 132 potential methylation sites in the progranulin locus (Banzhaf-Strathmann et al., 2013). Of these, five sites had slightly less methylation in people with the adenine TMEM106B locus, suggesting the variant might activate the PGRN gene.
“Their data and analysis provide key support for the hypothesis that TMEM106B variants are pathologically important and exert their influence upstream of expression of progranulin,” wrote Josephs and Nelson. Chen-Plotkin, who co-discovered the link between TMEM106B and FTLD, was pleased to see the effects on TMEM106B expression replicated in such a large cohort.
However, the results do not necessarily mean the TMEM106B genotype caused the progranulin overexpression, cautioned Rosa Rademakers and Dennis Dickson of the Mayo Clinic in Jacksonville, Florida, who were not part of the study. TDP-43 proteinopathy itself causes neuroinflammation and microglial activation, and this would be sufficient to upregulate progranulin, they said (Ahmed et al., 2007). Yu and colleagues are testing for activated microglia in the autopsied tissue. Rademakers added that studying progranulin methylation with respect to TMEM106B genotype was a novel line of inquiry, but she thought the data were not conclusive. “It does not seem to be a strong effect,” she said.
The pathway between TMEM106B and TDP-43 is likely complex, said Carlos Cruchaga of the Washington University School of Medicine in St. Louis. “Here we are only seeing the first steps of the network,” he added. Yu also expects that many more genes participate in that cascade, and wants to use more genome-wide analyses of people with TDP-43 proteinopathy to identify those additional players.—Amber Dance
References
News Citations
- The Four Stages of TDP-43 Proteinopathy
- TDP-43 Routes Mapped in Alzheimer’s, Frontotemporal Dementia
- Genetics of FTD: New Gene, PGRN Variety, and a Bit of FUS
- FTD Risk Factor Confirmed, Alters Progranulin Pathways
- Indianapolis: Dissecting the Pathways Behind Frontotemporal Dementia
Alzpedia Citations
Paper Citations
- Schwenk BM, Lang CM, Hogl S, Tahirovic S, Orozco D, Rentzsch K, Lichtenthaler SF, Hoogenraad CC, Capell A, Haass C, Edbauer D. The FTLD risk factor TMEM106B and MAP6 control dendritic trafficking of lysosomes. EMBO J. 2014 Mar 3;33(5):450-67. Epub 2013 Dec 19 PubMed.
- Stagi M, Klein ZA, Gould TJ, Bewersdorf J, Strittmatter SM. Lysosome size, motility and stress response regulated by fronto-temporal dementia modifier TMEM106B. Mol Cell Neurosci. 2014 Jul;61:226-40. Epub 2014 Jul 24 PubMed.
- Nicholson AM, Finch NA, Wojtas A, Baker MC, Perkerson RB, Castanedes-Casey M, Rousseau L, Benussi L, Binetti G, Ghidoni R, Hsiung GY, Mackenzie IR, Finger E, Boeve BF, Ertekin-Taner N, Graff-Radford NR, Dickson DW, Rademakers R. TMEM106B p.T185S regulates TMEM106B protein levels: implications for frontotemporal dementia. J Neurochem. 2013 Jun 6; PubMed.
- Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DM, . Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007 Jul;114(1):5-22. PubMed.
- Rutherford NJ, Carrasquillo MM, Li M, Bisceglio G, Menke J, Josephs KA, Parisi JE, Petersen RC, Graff-Radford NR, Younkin SG, Dickson DW, Rademakers R. TMEM106B risk variant is implicated in the pathologic presentation of Alzheimer disease. Neurology. 2012 Aug 14;79(7):717-8. PubMed.
- Nelson PT, Wang WX, Partch AB, Monsell SE, Valladares O, Ellingson SR, Wilfred BR, Naj AC, Wang LS, Kukull WA, Fardo DW. Reassessment of risk genotypes (GRN, TMEM106B, and ABCC9 variants) associated with hippocampal sclerosis of aging pathology. J Neuropathol Exp Neurol. 2015 Jan;74(1):75-84. PubMed.
- Aoki N, Murray ME, Ogaki K, Fujioka S, Rutherford NJ, Rademakers R, Ross OA, Dickson DW. Hippocampal sclerosis in Lewy body disease is a TDP-43 proteinopathy similar to FTLD-TDP Type A. Acta Neuropathol. 2015 Jan;129(1):53-64. Epub 2014 Nov 4 PubMed.
- Vass R, Ashbridge E, Geser F, Hu WT, Grossman M, Clay-Falcone D, Elman L, McCluskey L, Lee VM, Van Deerlin VM, Trojanowski JQ, Chen-Plotkin AS. Risk genotypes at TMEM106B are associated with cognitive impairment in amyotrophic lateral sclerosis. Acta Neuropathol. 2011 Mar;121(3):373-80. PubMed.
- Finch N, Carrasquillo MM, Baker M, Rutherford NJ, Coppola G, Dejesus-Hernandez M, Crook R, Hunter T, Ghidoni R, Benussi L, Crook J, Finger E, Hantanpaa KJ, Karydas AM, Sengdy P, Gonzalez J, Seeley WW, Johnson N, Beach TG, Mesulam M, Forloni G, Kertesz A, Knopman DS, Uitti R, White CL, Caselli R, Lippa C, Bigio EH, Wszolek ZK, Binetti G, Mackenzie IR, Miller BL, Boeve BF, Younkin SG, Dickson DW, Petersen RC, Graff-Radford NR, Geschwind DH, Rademakers R. TMEM106B regulates progranulin levels and the penetrance of FTLD in GRN mutation carriers. Neurology. 2011 Feb 1;76(5):467-74. PubMed.
- Banzhaf-Strathmann J, Claus R, Mücke O, Rentzsch K, van der Zee J, Engelborghs S, De Deyn PP, Cruts M, van Broeckhoven C, Plass C, Edbauer D. Promoter DNA methylation regulates progranulin expression and is altered in FTLD. Acta Neuropathol Commun. 2013 May 13;1(1):16. PubMed.
- Ahmed Z, Mackenzie IR, Hutton ML, Dickson DW. Progranulin in frontotemporal lobar degeneration and neuroinflammation. J Neuroinflammation. 2007;4:7. PubMed.
Further Reading
Papers
- Murray ME, Cannon A, Graff-Radford NR, Liesinger AM, Rutherford NJ, Ross OA, Duara R, Carrasquillo MM, Rademakers R, Dickson DW. Differential clinicopathologic and genetic features of late-onset amnestic dementias. Acta Neuropathol. 2014 Sep;128(3):411-21. Epub 2014 Jun 5 PubMed.
- Gallagher MD, Suh E, Grossman M, Elman L, McCluskey L, Van Swieten JC, Al-Sarraj S, Neumann M, Gelpi E, Ghetti B, Rohrer JD, Halliday G, Van Broeckhoven C, Seilhean D, Shaw PJ, Frosch MP, Alafuzoff I, Antonell A, Bogdanovic N, Brooks W, Cairns NJ, Cooper-Knock J, Cotman C, Cras P, Cruts M, De Deyn PP, DeCarli C, Dobson-Stone C, Engelborghs S, Fox N, Galasko D, Gearing M, Gijselinck I, Grafman J, Hartikainen P, Hatanpaa KJ, Highley JR, Hodges J, Hulette C, Ince PG, Jin LW, Kirby J, Kofler J, Kril J, Kwok JB, Levey A, Lieberman A, Llado A, Martin JJ, Masliah E, McDermott CJ, McKee A, McLean C, Mead S, Miller CA, Miller J, Munoz DG, Murrell J, Paulson H, Piguet O, Rossor M, Sanchez-Valle R, Sano M, Schneider J, Silbert LC, Spina S, van der Zee J, Van Langenhove T, Warren J, Wharton SB, White CL 3rd, Woltjer RL, Trojanowski JQ, Lee VM, Van Deerlin V, Chen-Plotkin AS. TMEM106B is a genetic modifier of frontotemporal lobar degeneration with C9orf72 hexanucleotide repeat expansions. Acta Neuropathol. 2014 Mar;127(3):407-18. Epub 2014 Jan 19 PubMed.
- van Blitterswijk M, Mullen B, Nicholson AM, Bieniek KF, Heckman MG, Baker MC, DeJesus-Hernandez M, Finch NA, Brown PH, Murray ME, Hsiung GY, Stewart H, Karydas AM, Finger E, Kertesz A, Bigio EH, Weintraub S, Mesulam M, Hatanpaa KJ, White CL 3rd, Strong MJ, Beach TG, Wszolek ZK, Lippa C, Caselli R, Petrucelli L, Josephs KA, Parisi JE, Knopman DS, Petersen RC, Mackenzie IR, Seeley WW, Grinberg LT, Miller BL, Boylan KB, Graff-Radford NR, Boeve BF, Dickson DW, Rademakers R. TMEM106B protects C9ORF72 expansion carriers against frontotemporal dementia. Acta Neuropathol. 2014 Mar;127(3):397-406. Epub 2014 Jan 3 PubMed.
- Busch JI, Martinez-Lage M, Ashbridge E, Grossman M, Van Deerlin VM, Hu F, Lee VM, Trojanowski JQ, Chen-Plotkin AS. Expression of TMEM106B, the frontotemporal lobar degeneration-associated protein, in normal and diseased human brain. Acta Neuropathol Commun. 2013 Jul 11;1(1):36. PubMed.
- Brady OA, Zheng Y, Murphy K, Huang M, Hu F. The frontotemporal lobar degeneration risk factor, TMEM106B, regulates lysosomal morphology and function. Hum Mol Genet. 2013 Feb 15;22(4):685-95. PubMed.
- Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol. 2007 May;61(5):435-45. PubMed.
- Nakashima-Yasuda H, Uryu K, Robinson J, Xie SX, Hurtig H, Duda JE, Arnold SE, Siderowf A, Grossman M, Leverenz JB, Woltjer R, Lopez OL, Hamilton R, Tsuang DW, Galasko D, Masliah E, Kaye J, Clark CM, Montine TJ, Lee VM, Trojanowski JQ. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol. 2007 Sep;114(3):221-9. PubMed.
- Cruchaga C, Graff C, Chiang HH, Wang J, Hinrichs AL, Spiegel N, Bertelsen S, Mayo K, Norton JB, Morris JC, Goate A. Association of TMEM106B gene polymorphism with age at onset in granulin mutation carriers and plasma granulin protein levels. Arch Neurol. 2011 May;68(5):581-6. PubMed.
- van der Zee J, Van Langenhove T, Kleinberger G, Sleegers K, Engelborghs S, Vandenberghe R, Santens P, Van den Broeck M, Joris G, Brys J, Mattheijssens M, Peeters K, Cras P, De Deyn PP, Cruts M, Van Broeckhoven C. TMEM106B is associated with frontotemporal lobar degeneration in a clinically diagnosed patient cohort. Brain. 2011 Mar;134(Pt 3):808-15. PubMed.
- Chen-Plotkin AS, Unger TL, Gallagher MD, Bill E, Kwong LK, Volpicelli-Daley L, Busch JI, Akle S, Grossman M, Van Deerlin V, Trojanowski JQ, Lee VM. TMEM106B, the risk gene for frontotemporal dementia, is regulated by the microRNA-132/212 cluster and affects progranulin pathways. J Neurosci. 2012 Aug 15;32(33):11213-27. PubMed.
Primary Papers
- Yu L, De Jager PL, Yang J, Trojanowski JQ, Bennett DA, Schneider JA. The TMEM106B locus and TDP-43 pathology in older persons without FTLD. Neurology. 2015 Mar 3;84(9):927-34. Epub 2015 Feb 4 PubMed.
- Josephs KA, Nelson PT. Unlocking the mysteries of TDP-43. Neurology. 2015 Mar 3;84(9):870-1. Epub 2015 Feb 4 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.