Do BACE Inhibitors Activate Microglia?
Quick Links
BACE inhibitors lower production of toxic Aβ peptides, and several such compounds are in clinical trials for people with prodromal or mild Alzheimer’s. Now, a new study suggests that these drugs could help later in disease, as well, and do so by an unexpected mechanism. In the April 30 Journal of Neuroscience, researchers at pharma companies Medtronic in Minneapolis and Bristol-Myers Squibb in Wallingford, Connecticut, report that two months of BACE inhibition in aged transgenic mice calmed inflammation and improved behavioral outcomes. Remarkably, treatment removed existing plaques, something that previously had been considered beyond the realm of drugs that turn down Aβ generation. This clearance correlated with microglial activation, though the mechanism remains mysterious.
Commentators were intrigued by the recovery in older mice. “Currently, we think that amyloid-lowering drugs may have their strongest effect in a preventive but not a therapeutic approach … [but] this study suggests that a BACE1 inhibitor may still be helpful at later stages in pathogenesis,” Stefan Lichtenthaler at the German Center for Neurodegenerative Diseases, Bonn, wrote to Alzforum (see full comment below).
However, Robert Vassar at Northwestern University, Evanston, Illinois, cautioned that the study is small and needs to be replicated. “The improvement in inflammatory markers was encouraging, but I would have liked to see more analysis to support the findings further,” he said. In the AD field, previous reports of plaque clearance in small mouse studies have often failed to replicate.
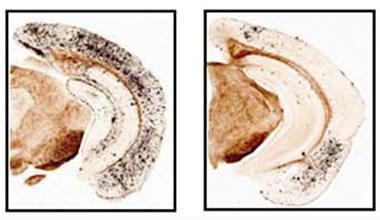
Amyloid Cleanup.
Heavy amyloid deposits (black) in the cortex and hippocampus of a 17-month-old transgenic mouse (left) dwindled after two months on the BACE inhibitor Compound C (right). [Image courtesy of Thakker et al., J.Neurosci, 2015.]
BACE inhibitors are known to slash production of Aβ in the brain, dropping cerebrospinal fluid (CSF) levels by as much as 80 percent in clinical trials (see Oct 2014 news; Apr 2015 conference news). While cognitive data is not yet available from human trials, in young transgenic mice the compounds improve cognition and prevent amyloid buildup.
The authors, led by Charles Albright at BMS and Lisa Shafer, then at Medtronic, wondered if BACE inhibitors could help mice that had already accumulated a heavy amyloid load. Joint first authors Deepak Thakker at Medtronic and Sethu Sankaranarayanan, then at BMS, tested the BMS BACE inhibitor, Compound C, in 17-month-old Tg2576 mice for eight weeks. Because the compound does not cross the blood-brain barrier, the authors administered various doses into the lateral ventricle of the brain via osmotic minipumps.
Similar to studies in younger animals, at the most effective dose of 1.1 μg/day, treatment cut soluble and insoluble brain Aβ levels almost in half and restored fear-conditioning responses to normal. Aβ did not change in cerebrospinal fluid.
Unexpectedly, at this dose plaque load dropped by half compared to baseline levels (see image above). This suggested that existing amyloid deposits were somehow being cleared. What might account for this? Measuring microglial activation with the marker CD68, the authors found a doubling in the cortex and hippocampus. Activated microglia clustered around plaques and had shorter, more ramified processes than those in control transgenics. Microglial activation correlated inversely and strongly with cortical plaque load (r2 = 0.9), suggesting the cells were mopping up amyloid. Other signs of inflammation, such as astrogliosis and number of dystrophic neurites, dropped by half as well.
Treatment produced none of the potential side effects researchers watch for with BACE inhibition or anti-amyloid treatment. Myelin thickness and grip strength remained normal, and the researchers saw no sign of microhemorrhages. At 1.1 μg/day, the death rate was similar to that in the control group, but at the highest dose of 23.5 μg/day, half the treated mice died, without additional plaque clearance. Deaths were likely due to off-target toxicity, since myelination remained unchanged in these mice, the authors noted.
The big unanswered question is how this BACE inhibitor might be switching on microglia, Vassar told Alzforum. No previous studies on this class of drugs have turned up any such connection. It is unclear if the finding would apply to other BACE inhibitors or if it is even related to BACE. Vassar noted that Compound C has been reported to inhibit Cathepsin D nearly as well as it blocks BACE1, raising the possibility that the microglial response is an off-target effect of this particular compound (see Meredith et al., 2008). Vassar was also puzzled by the fact that CSF Aβ did not change in the treated mice, contrary to the results from other preclinical work and ongoing clinical trials. Future studies should explore the mechanisms behind these findings, he suggested.
Bristol-Myers Squibb searched for BACE inhibitors for some years, but has discontinued this program (Marcin et al., 2011). The authors of the present paper declined multiple requests for comment.—Madolyn Bowman Rogers
References
News Citations
- Research Brief: New BACE Inhibitor Joins the Fold
- At AD/PD Meeting, New BACE Inhibitor Struts Its Stuff
Research Models Citations
Paper Citations
- Meredith JE, Thompson LA, Toyn JH, Marcin L, Barten DM, Marcinkeviciene J, Kopcho L, Kim Y, Lin A, Guss V, Burton C, Iben L, Polson C, Cantone J, Ford M, Drexler D, Fiedler T, Lentz KA, Grace JE, Kolb J, Corsa J, Pierdomenico M, Jones K, Olson RE, Macor JE, Albright CF. P-glycoprotein efflux and other factors limit brain amyloid beta reduction by beta-site amyloid precursor protein-cleaving enzyme 1 inhibitors in mice. J Pharmacol Exp Ther. 2008 Aug;326(2):502-13. PubMed.
- Marcin LR, Higgins MA, Zusi FC, Zhang Y, Dee MF, Parker MF, Muckelbauer JK, Camac DM, Morin PE, Ramamurthy V, Tebben AJ, Lentz KA, Grace JE, Marcinkeviciene JA, Kopcho LM, Burton CR, Barten DM, Toyn JH, Meredith JE, Albright CF, Bronson JJ, Macor JE, Thompson LA. Synthesis and SAR of indole-and 7-azaindole-1,3-dicarboxamide hydroxyethylamine inhibitors of BACE-1. Bioorg Med Chem Lett. 2011 Jan 1;21(1):537-41. PubMed.
Further Reading
News
- Seizures in BACE1 Knockouts: Are Potassium Channels to Blame?
- Scientists Suggest Curbing Sugar Modification to Tame BACE
- At High Doses, BACE1 Inhibitors Hinder Synaptic Plasticity in Mice
- Cloistered Retreat Takes the Pulse of BACE Research
- Merck BACE Inhibitor Clears a Safety Hurdle, Gets New Trial
- Lilly Teams Up With AstraZeneca for BACE Inhibitor Phase 2/3 Trial
Primary Papers
- Thakker DR, Sankaranarayanan S, Weatherspoon MR, Harrison J, Pierdomenico M, Heisel JM, Thompson LA, Haskell R, Grace JE, Taylor SJ, Albright CF, Shafer LL. Centrally Delivered BACE1 Inhibitor Activates Microglia, and Reverses Amyloid Pathology and Cognitive Deficit in Aged Tg2576 Mice. J Neurosci. 2015 Apr 29;35(17):6931-6. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
German Center for Neurodegenerative Diseases (DZNE)
I am particularly intrigued to see that the BACE1 inhibitor still has such a strong effect in old mice where amyloid pathology is already heavily visible. Currently, we think that amyloid-lowering drugs may have their strongest effect in a preventive but not a therapeutic approach. While we do not know whether a 17-month-old Tg2576 mouse (as tested here) corresponds to a presymptomatic or a symptomatic human individual, the study suggests that a BACE1 inhibitor may still be helpful at later stages in the pathogenesis. This supports testing BACE1 inhibitors not only in preventive trials but also in clinical trials with patients in early symptomatic phases, such as MCI (as currently underway).
View all comments by Stefan LichtenthalerMake a Comment
To make a comment you must login or register.