A Day in the OR: Surgeons Zap Neurons for Parkinson’s, AD
Quick Links
The doctors crowd around the computer monitor, examining the brain scans of the man lying on their operating table down the hall. The metal headdress they have mounted to his cranium—or “stereotactic frame” in medical lingo—provides coordinates, a sort of 3-D GPS they will use to guide platinum-iridium electrodes deep into his brain. The electricity pulsing through those electrodes will temporarily short out his misfiring globus pallidus. Hopefully, that will provide relief from the slow, abnormal movements caused not only by Parkinson’s disease, but also by the medications he has taken to control it for the past eight years. Deep-brain stimulation (DBS) has become a standard therapy for Parkinson’s and essential tremor (see ARF related news story); it is being tested now in a Phase 2 multicenter trial for AD (see below).
The 66-year-old man in the University of California, Los Angeles (UCLA) operating room is Daniel Duran of Rolling Hills Estates, California. Duran has had PD since 2004. Like many people with his condition, he noticed that medications no longer work well. He freezes up during long “off” periods between doses, and his “on” periods after a dose last for only 90 minutes. Having to be near a chair as every on period ends makes it hard for Duran, a retired engineer, to keep up his schedule of walks, workouts, support group meetings, and lunch dates. His state emulates that of Cinderella, recalled his wife Brenda Duran after the procedure—except “it is turning midnight every hour and a half.” Daniel has also been suffering side effects, including hallucinations that wake him, terrified, in the middle of the night. With today’s surgical procedure, Antonio De Salles, who heads UCLA’s DBS program, aims to improve Duran’s movement and reduce his medications.
Brenda and Daniel Duran hoped DBS surgery would stifle the symptoms of Parkinson’s and side effects from too much medication. Image courtesy of Daniel Duran
Before DBS was available, De Salles performed some 300 pallidotomies, destroying forever the area responsible for the poor movement control. DBS is preferable, he said, because it is reversible and the stimulation can be varied to find the level with the best effects. The voltage De Salles will shoot through the electrodes during the operation will shut off the globus pallidus much like a lesion would. “I am overwhelming the system with electrical stimulation,” De Salles said. The depolarized neurons should take a break, and Duran will see his muscle rigidity diminish within just a few minutes, right here in the operating room (OR). However, when the doctors turn off the voltage, the neurons can fire again, so unwanted side effects are not permanent, as they are with pallidotomies.
Duran’s day started at 3:30 a.m., when he and Brenda rose to go to the hospital. He was not allowed to eat, drink, or take his Parkinson’s meds before the surgery, so he arrived parched and stiff. He tried to convince every nurse who stopped by his room to give him his meds, because he is so uncomfortable without them. “He is like a drug addict,” Brenda joked. Daniel told her, “You don’t know what it’s like … it makes it a long day.” He has been in the operating room since about 7:30, under light anesthesia as the doctors formulate their plan.
Mapmaking
Before they cut, the surgical team must decide exactly where they want to place the electrodes, and what route they will take to reach the target tissue. They need to avoid blood vessels and pockets of cerebrospinal fluid (CSF). Since the larger vessels tend to nestle in the grooves of the brain’s cortex, the surgeons will enter the delicate organ away from those spaces. “You do not want to go through valleys; you want to go through mountains,” said Ausaf Bari, a neurosurgery resident on the case. “You can go through a lot of brain without hurting anything.”
And, of course, the physicians will forbear inserting the wires in Duran’s forehead, because that would not look handsome. For this kind of surgery, the insertion sites typically sit atop the head, one on each side—about where antennae on a Halloween headband might protrude.
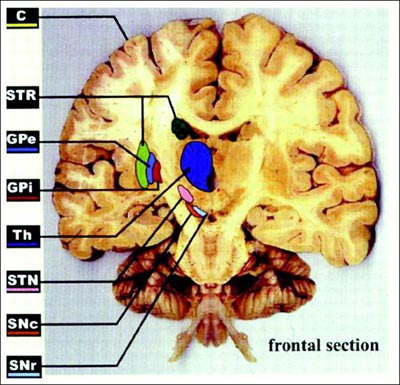
Today, De Salles has selected the internal globus pallidus (GPi) as the place to stimulate. The other option for Parkinson’s is the subthalamic nucleus (STN). Treatment in that area can be very effective, De Salles said; recipients typically cut their medication use by two-thirds. Targeting the GPi instead will probably mean that Duran can reduce his prescriptions by half, De Salles predicts, but it is a safer site for cognition. Older people with STN electrodes may become confused or aggressive. Thus, De Salles typically prefers the GPi for people who have passed 65. The GPi is also less risky for surgery, since it is larger and lies closer to the brain’s surface than does the STN (see ARF related news story on Follett et al., 2010, and ARF related news story on Weaver et al., 2012).
The frontal brain section illustrates DBS targets, including the subthalamic nucleus (STN) and internal globus pallidus (GPi). Image courtesy of Obeso et al., 2002. Reprinted with permission from The American Physiological Society.
At the computer, De Salles adjusts the plan to his liking, and Bari writes down the coordinates. He reads them back to make sure he made no mistake. “Let’s go do it, then,” De Salles says.
Go Time
Back in the OR—a large, white room with fluorescent lights and medical machinery—Michael Jackson’s “Smooth Criminal” plays quietly. Three monitors display Duran’s vitals. The doctors and nurses are careful not to bump against a blue-shrouded table holding their sterile instruments.
The surgeons, wearing blue scrubs and purple gloves, prepare for the operation. Five people adjust Duran’s head into just the right position for the surgery. Then, the anesthesiologist checks that he is still breathing easily. Bari shampoos his hair to minimize the risk of infection, since they are not shaving his entire scalp. Tufts of hair drop to the floor as they shave two spots. Bari draws the incision sites with a marker.
Next, the team adjusts a semicircular x-ray arm over Duran’s head. On each side of his head, they set up clear circles with crosshairs that will act as bullseyes for the electrodes. When the surgeons check their work, the electrode tips should appear right in the crosshairs on an x-ray image. Placing the x-ray targets takes time, but it is important. “Sometimes the little details make a big difference,” said Jacob Chodakiewitz, another surgeon on the team. The team hangs a plastic sheet from the x-ray arm, leaving just the top of Duran’s head exposed to the surgeons.
From the computed tomography scans taken earlier today, the doctors have determined the exact angle by which they will enter the brain with a guide tube holding the electrode. To find that angle, they use a large metal protractor mounted to the stereotactic frame on the man’s head.
Stereotactic frame as used in surgery to place deep-brain stimulation electrodes. Image courtesy of Elekta
The surgical nurse notes the time on a whiteboard as the doctors make the first cut. It is 10:24 a.m. They start on the left side of the head, making a small incision so they can see the bone underneath. The scalp tends to bleed a lot, so the surgeons quickly cauterize the tissue. They place a spreader to hold open the skin while the scrub nurse prepares the drill. It is smaller than a household drill, but the bit is long. The sound of the drill biting into the skull is surprisingly soft as Journey’s “Send Her My Love” plays in the background. Imagine a softer whine than the drill at your dentist’s office.
After all the planning and set up, inserting the electrode takes only a few minutes. The wire is live as the surgeons slide it in, and clinical specialist Eric Behnke calls out numbers as the electrode descends, “620 … 550 ….” He is measuring the resistance the tissue provides against the current, which tells the doctors where they are in the brain. CSF provides very little resistance; white matter, much more. By 10:30, it is time to wake up Duran and test the stimulation. The anesthesiologists turn off the propofol drip that has been keeping him sedated.
Open Your Eyes!
“Wake up!” Chodakiewitz yells—Duran is hard of hearing. “Open your eyes!” Duran moves his legs and picks at his blanket. Finally he mumbles, “I’m awake, I’m awake.”
The team will be looking for effects on the rigidity in Duran’s right hand, since they have operated on the left side of the brain. The main concern at this point is that the stimulation does not affect his thinking or cause other side effects. As Behnke ramps up the voltage—typical stimulation is 2 to 5 volts—Chodakiewitz asks Duran to count to 50 and sing, “Tie a Yellow Ribbon Round the Ole Oak Tree.” Duran succeeds in counting and warbling the chorus.
By 11:00, the doctors are satisfied that the stimulation is producing the desired effect without danger to other functions. The neuroanesthesiologist turns up the propofol again as the surgeons close the left incision and start on the right.
The second time Duran awakes, at 11:23, he knows the drill and offers up his right hand. Chodakiewitz gently takes the left instead for this round. Chodakiewitz notices that, as the voltage gets too high, Duran’s facial muscles contract. The GPi lies close to pathways that control the face, De Salles told Alzforum. Thus, seeing this side effect helped the doctors confirm the electrode was in the proper position (Gorgulho et al., 2009).
Once the team is satisfied with the second electrode’s stimulation, they let Duran drift back into the twilight of propofol sleep as they check the electrodes’ placement again on the x-ray and close the incision. They phone his wife and daughter to tell them the surgery went well. Later in the afternoon, around 4:00, Duran wakes up in the recovery room and demands his medication, telling nurses the drug names and precise dosages. At that point, Brenda Duran recalled later, she knew he must be okay.
The View From the Other Side of the Curtain
What is a DBS procedure like from the patient’s perspective? For his part, Duran remembers little from the OR, but Richard Thompson, a cartoonist in Arlington, Virginia, shared his story with Alzforum via e-mail, and with his fans via his blog. Thompson, who is 55, drew the syndicated comic strip Cul de Sac for five years; the final strip ran in September of 2012. Three years after his Parkinson’s diagnosis, he scheduled the operation for 12 October 2012. “It seemed like the next step that I should take in treatment, inevitable, so why put it off?” he wrote.
Without his medication, Thompson was wobbly when he got up at 4:00 a.m. to go to MedStar Georgetown University Hospital in Washington, DC. His wife and a friend helped him into the car. The empty streets on the chilly, foggy morning enhanced Thompson’s surreal impressions of the day, he wrote.
That mood intensified when he woke up in the operating room, mid-surgery, as the surgeons inserted the electrode. Lying with his head bolted to the stereotactic frame felt like “wearing a car grill,” he wrote. Overhearing his neurosurgeon chatting away on the other side of the plastic sheet draped around his head, Thompson was pleased to learn that he possesses a “robust thalamus.”
After placing the electrode, the doctors handed Thompson—still in the car grill—a pen and clipboard, as he had requested before the procedure. Without stimulation from the electrode now lodged in his brain, Thompson scratched out some squiggles meant to represent a brain and a spiral. The doctors turned on the juice and he tried again. Thompson could not see well without his glasses, but heard the medical team giggle at his drawings, so he figured the treatment had worked.
Cartoonist Richard Thompson penned this drawing—a brain saying, “Whee!”—during his DBS surgery, before (left) and after (right) the doctors turned on the juice. Image courtesy of Richard Thompson
“It was the strangest thing I had ever been through,” Thompson wrote. “Not altogether unpleasant, as I felt a bit spoiled by all the attention.”
A few months since the surgery, Thompson’s hair has grown back, and he was getting ready for his third programming session. At the first session, the effects of the stimulation were subtle, he told Alzforum. As the programmer adjusted the impulses, he might mumble or notice his smile go lopsided. The whole process took a few hours. But a week later, some of the shakiness returned. The second session was also a bit disappointing. “I’m hoping the third time is the charm,” he wrote.
“Wow! I Can Walk”
As for Duran, a few weeks after the electrode surgery, he returned to the hospital where De Salles implanted the impulse generator, which sends the stimulatory signals, into Duran’s chest. But Duran noticed benefits even before then. He and his wife thought he was imagining things when he saw, the morning after the first surgery, that his toes were not curled up tightly like on most mornings. In fact, De Salles expected something like this might happen. The GPi, having been prodded by the surgeons, was swollen and functioning poorly. Those relaxed toes were a good sign, indicating that disabling the GPi should reduce Duran’s symptoms.
This second surgery was shorter, but more difficult than the first, the Durans said. Duran acquired a series of staples marching down each side of his shaved scalp, and suffered more pain and a slower recovery. He underwent full anesthesia and did not know where he was when he woke up.
It was not until Duran had the stimulator turned on and programmed, at yet another appointment, that the benefits of DBS began to take full effect. His programmer held a remote control to his chest and amped the signal until Duran started tapping his feet and was able to traverse the hall with his walker. The effects felt the same as when his medication kicks in, Duran said.
The next morning, Duran hopped out of bed and walked into the bathroom unaided—he could barely make it there before the surgery. “Wow! I can walk … it’s real,” Duran told his wife. Brenda recalled, “We both just cried.”
Duran has dropped his daily pill count from 16 to 10, and hopes to go lower. His "on" periods last three to four hours, and he does not freeze up completely or fall over as the medications' effects wane. His only disappointment is that, just as he started to move better, the weather turned chilly, keeping him inside.
Memory Circuits
Could DBS one day become an equally accepted treatment for Alzheimer’s disease? Neurosurgeons have begun to test it. Unlike in Parkinson’s, where surgeons want to dampen undesirable signaling, doctors pursuing Alzheimer’s therapy want to increase activity. “It is like turning up the volume on a radio station,” said Andres Lozano of Toronto Western Hospital in Canada. Lozano is running a multicenter Phase 1 study, aiming to enroll 40 to 50 participants with mild AD to receive DBS to the fornix. “The fornix is the main pathway in and out of the memory circuit, so we thought that would be the best target,” he said.
In Lozano’s previous study of six people with AD, DBS was safe (see ARF related news story on Laxton et al., 2010). Some of the participants reported better memory and scored better on memory tests, but efficacy signals from such a small, open-label study mean little. One important finding from the pilot was that on positron emission tomography (PET) scans, the participants showed good glucose usage in the brain, said Constantine Lyketsos of Johns Hopkins University in Baltimore, Maryland. Normally, brain glucose metabolism decreases with progressing Alzheimer’s.
The Phase 2 study that Lozano and Lyketsos are now running is double-blinded; all subjects will receive electrodes, but only half will have them turned on. After a year, all participants will be able to receive stimulation. Lozano expects results from this study by 2015. He hopes that over 12 months, cognitive function will deteriorate more slowly in people with DBS than in those without. The team will also be investigating the rate of brain atrophy and blood flow.
Recruitment is going well, Lozano told Alzforum in an e-mail. His team in Toronto has operated on five patients since May 2012; Lyketsos’ Johns Hopkins group has performed two surgeries; and the Banner Alzheimer’s Institute in Phoenix, Arizona, has done one. The University of Florida in Gainesville will perform its first surgery this week. The Florida site has received some 40 inquiries from potential enrollees, according to Stacy Merritt at the university. “It is not easy to find the ideal candidate,” she wrote in an e-mail to Alzforum, because participants must fit into a specific range of cognitive and behavioral test scores. However, she is confident her site will enroll several subjects.
David Wolk and colleagues at the University of Pennsylvania in Philadelphia, another site, recently received institutional review board approval and are just getting started with recruitment. Brown University in Providence, Rhode Island, will also start enrolling subjects soon, according to Stephen Salloway at the University. Potential participants and their families find the concept of a “pacemaker for the brain” an appealing idea, he told Alzforum in an e-mail.
Lyketsos believes that the fornix is a good DBS target because it is affected early in the course of AD (Mielke et al., 2012); however, it is not the only possible bullseye for AD. Researchers at the University Hospital of Cologne, Germany, are testing DBS of the nucleus basalis of Meynert, one of the brain’s primary sources of acetylcholine. All current medications for Alzheimer’s work on the cholinergic system, noted Jens Kuhn, who is leading that study. He hopes to stimulate acetylcholine production with electricity, and thus improve memory and cognition. Kuhn is currently analyzing data from a pilot study of six people with mild AD, and expects to be able to conclude that the therapy is worthy of further investigation.
In their pilots, both Kuhn and Lozano noticed that younger people, with the mildest AD, saw the greatest benefit. Presumably, they had the most tissue left to save. In fact, DBS just might treat more than the symptoms of AD. Some studies have hinted that stimulation could cause growth factor production, Kuhn noted, which could help keep neurons alive. In a study of mice, Lozano and colleagues noticed that DBS enhanced neurogenesis in the hippocampus (see ARF related news story on Stone et al., 2011). “If this were to occur in humans, then there is a chance that the stimulation could alter the natural course of the illness,” Lozano said.—Amber Dance.
References
News Citations
- Deep-Brain Stimulation: Decade of Surgical Relief, Not Just for PD
- DBS Double Update: Call for Trial Registry, Two Targets Work for PD
- Zapping the Brain Can Help, But Where, and With What?
- DBS Update: Attempting to Stimulate Memory in Alzheimer’s
- Does Deep-Brain Stimulation Spark Neurogenesis, Enhance Learning?
Paper Citations
- Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, Marks WJ, Rothlind J, Sagher O, Moy C, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein JM, Stoner G, Starr PA, Simpson R, Baltuch G, De Salles A, Huang GD, Reda DJ, . Pallidal versus subthalamic deep-brain stimulation for Parkinson's disease. N Engl J Med. 2010 Jun 3;362(22):2077-91. PubMed.
- Weaver FM, Follett KA, Stern M, Luo P, Harris C, Hur K, Marks WJ, Rothlind J, Sagher O, Moy C. Randomized trial of deep brain stimulation for Parkinson disease: Thirty-six-month outcomes. Neurology. 2012 Jun 20;
- Obeso JA, Rodríguez-Oroz MC, Rodríguez M, Arbizu J, Giménez-Amaya JM. The basal ganglia and disorders of movement: pathophysiological mechanisms. News Physiol Sci. 2002 Apr;17:51-5. PubMed.
- Gorgulho AA, Shields DC, Malkasian D, Behnke E, Desalles AA. Stereotactic coordinates associated with facial musculature contraction during high-frequency stimulation of the subthalamic nucleus. J Neurosurg. 2009 Jun;110(6):1317-21. PubMed.
- Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, Wherrett J, Naglie G, Hamani C, Smith GS, Lozano AM. A phase I trial of deep brain stimulation of memory circuits in Alzheimer's disease. Ann Neurol. 2010 Oct;68(4):521-34. PubMed.
- Mielke MM, Okonkwo OC, Oishi K, Mori S, Tighe S, Miller MI, Ceritoglu C, Brown T, Albert M, Lyketsos CG. Fornix integrity and hippocampal volume predict memory decline and progression to Alzheimer's disease. Alzheimers Dement. 2012 Mar;8(2):105-13. PubMed.
- Stone SS, Teixeira CM, Devito LM, Zaslavsky K, Josselyn SA, Lozano AM, Frankland PW. Stimulation of entorhinal cortex promotes adult neurogenesis and facilitates spatial memory. J Neurosci. 2011 Sep 21;31(38):13469-84. PubMed.
Other Citations
External Citations
Further Reading
Papers
- Laxton AW, Lozano AM. Deep Brain Stimulation for the Treatment of Alzheimer Disease and Dementias. World Neurosurg. 2012 Jun 19; PubMed.
- Okun MS. Deep-brain stimulation for Parkinson's disease. N Engl J Med. 2012 Oct 18;367(16):1529-38. PubMed.
- Castrioto A, Lozano AM, Poon YY, Lang AE, Fallis M, Moro E. Ten-year outcome of subthalamic stimulation in Parkinson disease: a blinded evaluation. Arch Neurol. 2011 Dec;68(12):1550-6. PubMed.
- Hamani C, McAndrews MP, Cohn M, Oh M, Zumsteg D, Shapiro CM, Wennberg RA, Lozano AM. Memory enhancement induced by hypothalamic/fornix deep brain stimulation. Ann Neurol. 2008 Jan;63(1):119-23. PubMed.
News
- Virtual Reality Reveals Deep-Brain Memory Stimulus, Early AD Signs
- Meynert, Oh, My! Deep Brain Stimulation to Treat Dementia?
- Deep-Brain Stimulation: Decade of Surgical Relief, Not Just for PD
- DBS Double Update: Call for Trial Registry, Two Targets Work for PD
- Zapping the Brain Can Help, But Where, and With What?
- DBS Update: Attempting to Stimulate Memory in Alzheimer’s
- Deep-Brain Stimulation Improves Connectivity in AD
- DBS Draws Consensus Nod for Parkinson’s, But Not Other Conditions
- PD Studies Highlight Deep Brain Stimulation, New Role for α-Synuclein
- Does Deep-Brain Stimulation Spark Neurogenesis, Enhance Learning?
Annotate
To make an annotation you must Login or Register.




Comments
No Available Comments
Make a Comment
To make a comment you must login or register.