Brain Spheroids Hatch Mature Astrocytes
Quick Links
Astrocytes are more than bystanders in neurotransmission—they take an active role in synaptic activity. However, their functions are hard to study because the cells are difficult to grow in vitro and it’s hard to coax them to mature from progenitors. Now, researchers from the labs of Sergiu Paşca and Ben Barres, both at Stanford University School of Medicine, California, report that astrocytes come of age in spherical balls of human brain cells cultured in a dish for almost two years. As reported in the August 16 Neuron, these astrocytes develop much like those from real brains, undergoing similar transcriptomic, morphologic, and functional changes. Studying the processes involved in this astrocyte maturation will help researchers understand neurodevelopmental disorders such as autism and schizophrenia, researchers say, and might even shed light on problems in adult brains.
“That these three-dimensional cultures can be maintained for such a long time allows us to capture an interesting transition in astrocytes,” said Paşca. “We are starting to appreciate aspects of human brain development to which we would not otherwise have access.”
“The breakthrough is that they can develop human astrocytes very close to maturity in their three-dimensional culture models,” said Doo Yeon Kim, Massachusetts General Hospital, Charlestown, who uses three-dimensional culture models to study pathological process that occur in Alzheimer’s disease. Some researchers are using three-dimensional cultures to model other neurodegenerative disorders, such as ALS, and still others are planning to use cultured astrocytes for cell therapy. “If astrocytes are not mature enough in culture, patterns [we see] may not be the same as in the diseased brain,” said Kim.
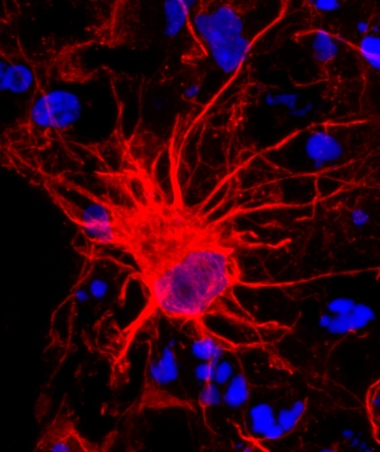
Approaching maturity
This developing human astrocyte (red), which comes from a 350-day-old cortical spheroid, is taking shape as a mature cell. [Image courtesy of Sloan et al. Neuron]
A few years back, Paşca’s group developed a method for differentiating human-induced pluripotent stem cells (hiPSCs) into a three-dimensional culture of brain cells. They used special dishes that the cells could not easily attach to, coaxing them to stick to each other instead. Under these conditions the iPSCs balled up into neural spheroids that grew to about 4 mm in diameter. A cocktail of growth factors early on encouraged them to form excitatory pyramidal cells like those in the cortex, and the cells spontaneously organized into layers. These cortical spheroids survived a year or more and spontaneously grew astrocytes in addition to neurons (Paşca et al., 2015). Not long after, the Barres lab reported that astrocytes in the adult human brain look different from those isolated from fetuses. They called the latter astrocyte progenitor cells (APCs). Each had its own transcriptional patterns and functions (Jan 2016 news). Together, Barres and Paşca wondered if it was possible to see the APCs morph into mature astrocytes in these long-lived cortical spheroids.
To find out, first author Steven Sloan and colleagues examined spheroids generated from iPSCs derived from healthy human fibroblasts. Sloan grew the spheroids for about 20 months. Along the way, he took samples, isolated the astrocytes, and compared them to those isolated from fetal and postnatal human brains.
At about 100 days in culture, astrocytes began to sprout spontaneously from within the mostly neuronal milieu of the cortical spheroids. At first, these cells were simple, adorned by few branches and expressing genes akin to those active in APCs. But as the spheroids reached about 250 days, the astrocytes therein looked more mature, having numerous processes. After this point, APC gene expression tapered off and the astrocytes started producing proteins typical of mature astrocytes.
Astrocytes also underwent functional changes as they matured. Early versions divided in fast and furious fashion, much like their counterparts from the fetal tissue. That division slowed as the spheroids aged. Dividing APCs dropped from 35 percent of all astrocytes at day 167 to 3 percent at day 590. Taken from the spheroids at day 150 and cultured in a two-dimensional layer, immature astrocytes also harbored a voracious appetite for added synaptosomes, much like immature astrocytes recently characterized in mice (see image below; Dec 2013 conference news on Chung et al., 2013). However, that hunger waned as astrocytes approached the 590-day mark.
At the older end of the spectrum, mature astrocytes seemed to take on a supportive role, strengthening calcium signaling in nearby neurons.
Studying the neurons and astrocytes in these cortical spheroids could be useful for addressing certain unanswered questions about human biology, said other researchers. “This could be a very strong opportunity to understand what goes wrong in human genetic disorders that affect astrocyte function,” said M. Kerry O’Banion, University of Rochester Medical Center, New York. It’s also possible that such cultures could reveal as-yet-unknown facets of familial mutations that cause Alzheimer’s disease, he suggested. However, given that these cultures take a long time to grow and develop, they are unlikely to completely supplant other types of cultures or faster-maturing animal models, he said.
Kim agreed, saying, “The results are very exciting, but not practical yet for disease modeling.” However, Kim hopes that researchers will make progress on accelerating the maturation process.
The Barres and Paşca labs are trying just that with the spheroid. They will also analyze what they secrete to support neuronal signaling. In addition, they are exploring how to make the astrocytes reactive, as they often are in neurodegenerative diseases, such as Alzheimer’s. Doing so might reveal how such astrocytes interact with neurons.
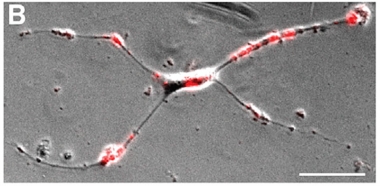
Young and Hungry
An immature astrocyte taken from a 150-day-old spheroid gobbles up added synaptosomes (red). [Neuron, Sloan et al. 2017]
To Paşca’s knowledge, these cortical spheroids are some of the longest human cell cultures ever reported. His group has continued to cultivate these clumps, with the oldest still going strong at day 850. Granted, these systems are missing many cell types: endothelial cells, oligodendrocytes, and microglia to name a few, he said. However, his lab has introduced new ways to add in other cells. Earlier this year, he reported three-dimensional cultures of cortical glutamatergic neurons and GABAergic interneurons that fused together when they were placed side-by-side (Birey et al., 2017).
Clive Svendsen, Cedars-Sinai Medical Center in Los Angeles, California, saw clinical implications for this paper. “It shows iPSC-derived astrocytes can mature to an adult phenotype,” he said. This further supports their use in clinical transplantation, as we are planning to do.” His group has begun a Phase 1 clinical trial that implants human fetal astrocytes into the spinal cords of ALS patients.—Gwyneth Dickey Zakaib
References
News Citations
Paper Citations
- Paşca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, Kim CH, Park JY, O'Rourke NA, Nguyen KD, Smith SJ, Huguenard JR, Geschwind DH, Barres BA, Paşca SP. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods. 2015 Jul;12(7):671-8. Epub 2015 May 25 PubMed.
- Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, Smith SJ, Barres BA. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013 Dec 19;504(7480):394-400. Epub 2013 Nov 24 PubMed.
- Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N, Fan HC, Metzler KR, Panagiotakos G, Thom N, O'Rourke NA, Steinmetz LM, Bernstein JA, Hallmayer J, Huguenard JR, Paşca SP. Assembly of functionally integrated human forebrain spheroids. Nature. 2017 May 4;545(7652):54-59. Epub 2017 Apr 26 PubMed.
External Citations
Further Reading
Papers
- Dezonne RS, Sartore RC, Nascimento JM, Saia-Cereda VM, Romão LF, Alves-Leon SV, de Souza JM, Martins-de-Souza D, Rehen SK, Gomes FC. Derivation of Functional Human Astrocytes from Cerebral Organoids. Sci Rep. 2017 Mar 27;7:45091. PubMed.
- Brawner AT, Xu R, Liu D, Jiang P. Generating CNS organoids from human induced pluripotent stem cells for modeling neurological disorders. Int J Physiol Pathophysiol Pharmacol. 2017;9(3):101-111. Epub 2017 Jun 15 PubMed.
Primary Papers
- Sloan SA, Darmanis S, Huber N, Khan TA, Birey F, Caneda C, Reimer R, Quake SR, Barres BA, Paşca SP. Human Astrocyte Maturation Captured in 3D Cerebral Cortical Spheroids Derived from Pluripotent Stem Cells. Neuron. 2017 Aug 16;95(4):779-790.e6. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
UCL Institute of Neurology and The Francis Crick Institute
This study has addressed a salient issue in human astrocyte biology. It nicely confirms and extends a previous study by the Barres Lab (Zhang et al., 2016), which characterized purified astrocytes from fetal through to adult stages. It chronicles the transcriptional progression, and acquisition of different functional attributes, during astrocytic maturation using a translational and reductionist human iPSC model.
These findings will serve as a powerful resource for researchers studying astrocyte developmental biology and also issues of cellular autonomy in a range of neurodegenerative disorders.
References:
Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MS, Li G, Duncan JA 3rd, Cheshier SH, Shuer LM, Chang EF, Grant GA, Gephart MG, Barres BA. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron. 2016 Jan 6;89(1):37-53. Epub 2015 Dec 10 PubMed.
Make a Comment
To make a comment you must login or register.