Asthma Drug Revitalizes Memory in Old Rodent Brains
Quick Links
A drug that opens constricted airways in asthma sufferers may also revitalize aged brains. As reported October 27 in Nature Communications, montelukast, an inhibitor of leukotriene receptors that mediate inflammatory responses, boosted the growth of new neurons, reduced neuroinflammation, and reinforced the blood-brain barrier in aged rats. Importantly, the drug also restored learning and memory in the rodents, a benefit that correlated strongly with the rate of neurogenesis stimulated by the treatment. The researchers, led by Ludwig Aigner at Paracelsus Medical University in Salzburg, Austria, reported that the proliferation of new neurons by montelukast was mediated through its engagement of GPR17, a leukotriene receptor expressed primarily on neuronal cells. They plan to study the effects of the drug in animal models of neurodegenerative disease, and ultimately in clinical trials.
“That montelukast was already approved for asthma should speed up the drug-development process for its use in aging and neurodegenerative disease,” Aigner told Alzforum. The findings underscore the presence of fundamental regenerative mechanisms across multiple organs in the body, he added.
As our brains age, they accumulate many changes that could contribute to cognitive fuzziness. The production of new neurons slows down, neuroinflammation increases, synapses lose their vigor, and the integrity of the blood-brain barrier may weaken. What if a single treatment could stem all of these age-related problems in the brain? Previous studies that reported the blood of young mice revitalized brain function in old mice (while blood from old mice did the opposite for young recipients) suggested that such factors may exist (see Aug 2011 news; May 2014 conference news). These researchers, led by Tony Wyss-Coray at Stanford University in Palo Alto, California, reported that a chemokine called eotaxin in the blood of old mice reduced neurogenesis, increased neuroinflammation, and taxed cognition in young mice. Eotaxin mediates inflammatory responses in people with asthma, so Aigner and colleagues wondered whether mechanisms involved in peripheral inflammatory conditions such as asthma might promote brain aging.
They turned to leukotrienes, inflammatory mediators that promote asthmatic and other inflammatory reactions in the body (see Peters-Golden, 2008). What leukotrienes do in the brain is unclear, but the molecules and their receptors are reportedly elevated following stroke, near CNS lesions, and in the aging brain (see Farias et al., 2009; Chinnici et al., 2007). Leukotrienes have been reported to increase amyloid production and cognitive decline in AD mouse models, while blocking their action protects neurons from Aβ toxicity (see Tang et al., 2013; Lai et al., 2014; and Tang et al., 2014). Leukotriene receptor antagonists, such as montelukast, dampen inflammation and have been used to successfully treat asthma patients (see Reiss et al., 1996). In addition to its anti-inflammatory effects, montelukast boosts the proliferation of neural progenitor cells in adult neurospheres, according to a previous study from Aigner’s lab (see Huber et al., 2011).
For the current study, first author Julia Marschallinger and colleagues sought to test whether montelukast would boost flagging neurogenesis and calm elevated neuroinflammation in aged rats, and whether this could make the aging animals sharp again. The researchers fed montelukast to both young (four-month-old) and aged (20-month-old) rats for six weeks, at a dose similar to what asthma patients take. They found that old rats took longer to learn the location of a submerged platform in the Morris water maze test than their younger counterparts did, but that montelukast treatment elevated their performance. After five days of training, old rats treated with montelukast found the platform as quickly as treated or untreated young rats. The treatment also gave the old rats more detailed spatial memory, as the animals spent more time in the exact location, rather than just the general vicinity, of a previously hidden platform after it was removed.
The researchers next examined how well oral montelukast entered the brain, given reports that the BBB may lose its integrity with age. They tracked montelukast levels in the blood and CSF of rats as well as in one person who took a similar dose. They found the drug in the blood and CSF of both young and aged animals, as well as in the one human participant. Despite its entry into the brain, montelukast also appeared to enhance the blood-brain barrier. The researchers observed elevated and continuous staining of claudin-5, a tight junction protein expressed by endothelial cells that line the barrier, in aged animals.
To assess the effects of montelukast on neuroinflammation, the researchers examined microglia, the macrophages of the brain. They found that compared to young animals, aged rats had microglia with larger cell bodies, a sign of activation. The cells also expressed higher levels of the phagocytic marker CD68, and contained larger intracellular CD68-bearing particles than those in younger rats. Treatment with montelukast reduced the size of microglial cell bodies as well as the size of CD68-bearing particles, indicating that the drug may soothe agitated microglia in the aging brain.
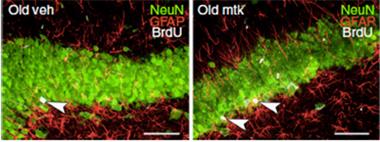
Neuronal Awakenings?
Old rats treated with montelukast (right) contain more recently divided neurons (white/green) in the dentate gyrus than untreated animals (left). [Courtesy of Marschallinger et al., Nature Communications 2015.]
Neurogenesis also increased on montelukast. The researchers report that aged rats had fewer dividing cells in the dentate gyrus of the hippocampus than their younger counterparts, and treatment with montelukast restored this proliferation. The new cells resulting from this growth were mostly neurons. Because all rats underwent behavioral studies prior to analysis of their brains, the researchers could determine which cellular effects correlated most closely with boosts in learning. They found that the cognitive benefits of montelukast in aged rats did not correlate with microglial morphology or size, but rather with the number of proliferating cells in the dentate gyrus. This suggested that the boost in neurogenesis was the primary driver of the learning benefits. However, Aigner pointed out that a more extensive analysis of neuroinflammation needs to be conducted, and also that neuroinflammation may play an important role in other contexts, such as during neurodegenerative disease.
Why did montelukast target neurogenic regions of the brain? One clue was that 5-LOX, the key enzyme in leukotriene synthesis, was highly elevated in those niches in the hippocampus in aged, but not young, rats. Based on immunohistochemistry of human postmortem brain samples, the researchers also found that people older than 60 had higher levels of the enzyme in the dentate gyrus than did people younger than 35. Furthermore, the researchers found that neuronal progenitors and some microglia in the dentate gyrus expressed the leukotriene receptor GPR17, while CysLTR1, another leukotriene receptor, was found primarily on glial cells. Further homing in on GPR17, the researchers generated neurosphere cultures from mice lacking either GPR17 or Foxo1, the transcription factor necessary for GPR17 expression. In both cases, proliferation of neural progenitor cells in the cultures increased significantly, and adding montelukast did nothing to enhance this effect. In contrast, Foxo1 overexpression reduced proliferation. These results suggested that the neurogenic effect of montelukast was mediated through antagonism of GPR17.
“This study has identified leukotriene receptors in the brain as a novel anti-aging target, and hopefully future work will divulge whether this can slow symptoms of age-associated neurodegenerative diseases as well,” commented Melanie Das of Cedars-Sinai Medical Center in Los Angeles. She added that it will be important to investigate the effects of montelukast on the function of glial cells in the neurogenic niche and elsewhere in the brain, as those cells also play an important role in cognitive decline.
Aigner told Alzforum that his group is currently investigating whether montelukast improves cognition in animal models of neurodegenerative disease. In parallel with more basic research, the researchers are also seeking funding for clinical trials in people with neurodegenerative disease.
“The data are compelling, and given the widespread use of the drug to date, a small clinical trial addressing acute improvements in cognition in human subjects could be interesting and worthwhile,” commented Matthew Campbell of Trinity College in Dublin. In agreement with Aigner, Campbell also proposed testing the drug in people with cognitive impairments that go beyond healthy aging.—Jessica Shugart
References
News Citations
- Paper Alert: Do Blood-Borne Factors Control Brain Aging?
- In Revival of Parabiosis, Young Blood Rejuvenates Aging Microglia, Cognition
Paper Citations
- Peters-Golden M. Expanding roles for leukotrienes in airway inflammation. Curr Allergy Asthma Rep. 2008 Jul;8(4):367-73. PubMed.
- Farias S, Frey LC, Murphy RC, Heidenreich KA. Injury-related production of cysteinyl leukotrienes contributes to brain damage following experimental traumatic brain injury. J Neurotrauma. 2009 Nov;26(11):1977-86. PubMed.
- Chinnici CM, Yao Y, Praticò D. The 5-lipoxygenase enzymatic pathway in the mouse brain: young versus old. Neurobiol Aging. 2007 Sep;28(9):1457-62. Epub 2006 Aug 22 PubMed.
- Tang SS, Wang XY, Hong H, Long Y, Li YQ, Xiang GQ, Jiang LY, Zhang HT, Liu LP, Miao MX, Hu M, Zhang TT, Hu W, Ji H, Ye FY. Leukotriene D4 induces cognitive impairment through enhancement of CysLT₁ R-mediated amyloid-β generation in mice. Neuropharmacology. 2013 Feb;65:182-92. Epub 2012 Sep 12 PubMed.
- Lai J, Mei ZL, Wang H, Hu M, Long Y, Miao MX, Li N, Hong H. Montelukast rescues primary neurons against Aβ1-42-induced toxicity through inhibiting CysLT1R-mediated NF-κB signaling. Neurochem Int. 2014 Sep;75:26-31. Epub 2014 May 28 PubMed.
- Tang SS, Hong H, Chen L, Mei ZL, Ji MJ, Xiang GQ, Li N, Ji H. Involvement of cysteinyl leukotriene receptor 1 in Aβ1-42-induced neurotoxicity in vitro and in vivo. Neurobiol Aging. 2014 Mar;35(3):590-9. Epub 2013 Oct 23 PubMed.
- Reiss TF, Altman LC, Chervinsky P, Bewtra A, Stricker WE, Noonan GP, Kundu S, Zhang J. Effects of montelukast (MK-0476), a new potent cysteinyl leukotriene (LTD4) receptor antagonist, in patients with chronic asthma. J Allergy Clin Immunol. 1996 Sep;98(3):528-34. PubMed.
- Huber C, Marschallinger J, Tempfer H, Furtner T, Couillard-Despres S, Bauer HC, Rivera FJ, Aigner L. Inhibition of leukotriene receptors boosts neural progenitor proliferation. Cell Physiol Biochem. 2011;28(5):793-804. Epub 2011 Dec 15 PubMed.
Further Reading
Papers
- Joshi YB, Praticò D. The 5-lipoxygenase pathway: oxidative and inflammatory contributions to the Alzheimer's disease phenotype. Front Cell Neurosci. 2014;8:436. Epub 2015 Jan 14 PubMed.
- Tang SS, Ji MJ, Chen L, Hu M, Long Y, Li YQ, Miao MX, Li JC, Li N, Ji H, Chen XJ, Hong H. Protective effect of pranlukast on Aβ₁₋₄₂-induced cognitive deficits associated with downregulation of cysteinyl leukotriene receptor 1. Int J Neuropsychopharmacol. 2014 Apr;17(4):581-92. Epub 2013 Nov 11 PubMed.
Primary Papers
- Marschallinger J, Schäffner I, Klein B, Gelfert R, Rivera FJ, Illes S, Grassner L, Janssen M, Rotheneichner P, Schmuckermair C, Coras R, Boccazzi M, Chishty M, Lagler FB, Renic M, Bauer HC, Singewald N, Blümcke I, Bogdahn U, Couillard-Despres S, Lie DC, Abbracchio MP, Aigner L. Structural and functional rejuvenation of the aged brain by an approved anti-asthmatic drug. Nat Commun. 2015 Oct 27;6:8466. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Cedars-Sinai Medial Center
A short-term oral treatment of the anti-inflammatory drug montelukast was enough for significant cognitive improvement in naturally aged rats. Along with a reduction of neuroinflammation, the authors found that montelukast increased neurogenesis, improved blood-brain barrier (BBB) integrity, and improved cognition. Whether a reduction in inflammation directly contributed to these other improvements or whether these are independent effects of montelukast is unknown. However, the rejuvenating effects of montelukast are consistent with the recent line of parabiosis studies showing that young blood-borne factors can improve cognition, increase neurogenesis, and decrease microglia activation in aged mice (Villeda et al., 2011). In both cases, short-term treatment regimens are sufficient to see significant improvements in cognition and neurogenesis and decreases in inflammation. As with the blood studies, it is of interest to further investigate the dynamics and long-term effects of montelukast. For example, does montelukast turn on a switch in various brain cells, thus changing their phenotype and resulting in long-lasting cognitive effects? Or does cognitive improvement quickly decline when treatment is halted?
The authors focused on the hippocampal neural progenitors and neurons in this study, but the montelukast targets, CysLTR1 and GPR17, are also expressed in non-neuronal cells, suggesting other possible mechanisms by which brain rejuvenation occurs. Since glial cells, especially microglia and astrocytes, have been shown to directly contribute to cognitive decline it will be extremely interesting to understand the impact of montelukast on glial cells in the neurogenic niche and elsewhere throughout brain (Sierra et al., 2014; Miranda et al., 2012).
So far, anti-inflammatory drugs have met with limited success in clinical trials of various neurodegenerative diseases. However, this study exciting because i) montelukast is already a clinically approved drug and ii) it crosses the BBB.
This study has identified leukotriene receptors in the brain as a novel anti-aging target and hopefully future work will divulge whether this can slow symptoms of age-associated neurodegenerative diseases as well.
References:
Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Després S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011 Sep 1;477(7362):90-4. PubMed.
Sierra A, Beccari S, Diaz-Aparicio I, Encinas JM, Comeau S, Tremblay ME. Surveillance, Phagocytosis, and Inflammation: How Never-Resting Microglia Influence Adult Hippocampal Neurogenesis. Neural Plast. 2014;2014:610343. Epub 2014 Mar 19 PubMed.
Miranda CJ, Braun L, Jiang Y, Hester ME, Zhang L, Riolo M, Wang H, Rao M, Altura RA, Kaspar BK. Aging Brain Microenvironment Decreases Hippocampal Neurogenesis Through Wnt-Mediated Survivin Signaling. Aging Cell. 2012 Mar 8; PubMed.
Make a Comment
To make a comment you must login or register.