ApoE4 Traps Insulin Receptor Inside Neurons
Quick Links
Scientists led by Guojun Bu, Mayo Clinic, Jacksonville, Florida, believe they have found an important link between the E4 isoform of apolipoprotein E and insulin resistance in the brain. In the September 27 Neuron, they report that ApoE4 competes with the hormone for binding to its receptor on neurons, leading to insulin resistance. To make matters worse, ApoE4 then ensnares insulin receptors in early endosomes, preventing their speedy return to the cell surface. Clogging up, then sequestering, insulin receptors unleashes a double whammy on normal insulin signaling, the scientists show.
- In aged and in diabetic mice, ApoE4 traps the insulin receptor in early endosomes.
- This dampens insulin signaling in the brain.
- The authors have yet to show that ApoE4 works the same way in people.
“This represents a significant advance in our knowledge about mechanisms underlying the effects of ApoE4 and insulin resistance in the brain,” wrote Suzanne Craft, Wake Forest School of Medicine, Winston-Salem, North Carolina, to Alzforum.
Normally, insulin binds its receptor on the surface of neurons, then promptly undergoes endocytosis to end up in early endosomes. This process triggers glycolysis in the neurons. Much of the insulin receptor then recycles back up to the cell surface for more signaling. In the AD brain, cells can be less responsive to insulin (Steen et al., 2005). When researchers try boosting insulin signaling in the brain by delivering insulin through the nose, ApoE4 carriers respond poorly compared with noncarriers with regular insulin, but do better than noncarriers with a longer-lasting formulation (Craft et al., 2017). How does ApoE genotype affect insulin signaling in the brain?
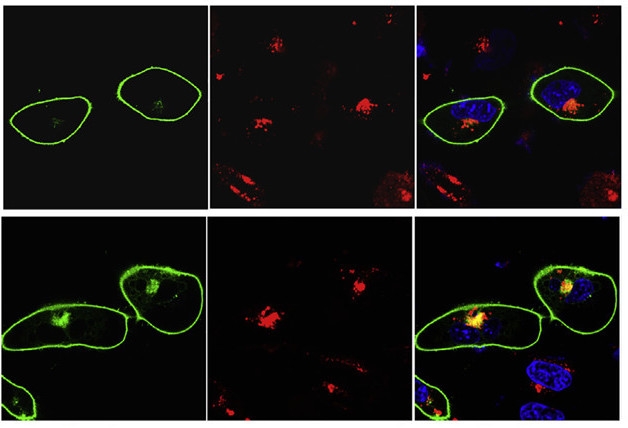
Stuck in Endosome. ApoE-/- neurons (green outline, top and bottom rows) treated with ApoE3 (top row) and ApoE4 (bottom). Bottom shows insulin receptors (green dots) co-localizing (yellow) with early endosomes (red). [Courtesy of Neuron, Zhao et al.]
To address this question mechanistically, co-first authors Na Zhao and Chia-Chen Liu studied how insulin resistance differed in mice that had their own ApoE gene replaced by either the human ApoE3 or the E4 allele, aka targeted replacement (TR) mice. Zhao and colleagues examined them at three, 12, and 22 months of age. In youth and middle age, endogenous insulin elicited a similar downstream response in ApoE4 and in ApoE3 mice. However, 22-month-old ApoE4 mice responded poorly to endogenous insulin compared to ApoE3 mice. These older ApoE4 mice also responded more weakly to insulin delivered into the brain by reverse microdialysis.
The findings indicate that ApoE4 suppresses insulin signaling in old TR mice. It had similar effects in diabetic animals. When the researchers induced diabetes with a high-fat diet, ApoE4- but not ApoE3-TR mice responded weakly to endogenous and administered insulin at 12 months of age. Overall, the results suggest that ApoE4 causes insulin resistance in the brain as mice age, and that diabetes hastens this age effect.
To study what might be happening at the molecular level, Zhao and colleagues cultured mouse primary neurons. They treated them with human ApoE3 or ApoE4, then added insulin. In the brain, neurons normally encounter ApoE extracellularly, as made and released by astrocytes. Control ApoE-/- neurons responded robustly to insulin, ramping up both glycolysis and mitochondrial respiration. Ditto for ApoE3-treated neurons. In contrast, ApoE4 dampened both types of metabolism.
Did ApoE4 come between insulin and its receptor? In a binding assay, both recombinant ApoE3 and ApoE4 bound the insulin receptor, but ApoE4’s affinity was higher. In response to added insulin, fewer insulin receptors in ApoE4-treated neurons endocytosed than did in ApoE3-treated neurons, suggesting ApoE4 competed with insulin to prevent receptor trafficking. However, once the receptor made it to the endosomes, it got stuck there in ApoE4-treated cells (see image above). A more physiologically relevant source of ApoE came from astrocyte-conditioned medium. Both ApoE3 and ApoE4 had 10 times higher affinity for the insulin receptor than their respective recombinant proteins, but again only ApoE4 dampened insulin signaling.
When the researchers checked in brain lysates from TR mice, they detected an interaction between ApoE4 and the insulin receptor in the brains of old animals, but not young ones. Moreover, ApoE4 aggregated more in aged than in young mice, suggesting some large form of the apolipoprotein might be causing problems in older mice. Bu told Alzforum that when ApoE4 aggregates, it clumps with other proteins, such as the insulin receptor, in early endosomes. This would prevent the receptor, and possibly other signaling molecules, from returning to the cell surface. In support of this, the scientists saw that the insulin receptor, too, aggregated in older animals.
ApoE4, the strongest genetic risk factor for late-onset Alzheimer’s, interacts with Aβ and affects its ability to aggregate (Jan 2017 news; Apr 2013 news). Bu plans to test if Aβ worsens ApoE4-induced insulin resistance, and how ApoE genotype affects insulin sensitivity in mouse models of amyloidosis. The scientists have yet to show that ApoE4 interacts with the insulin receptor in people. Craft’s current Phase 2/3 trial, in which AD patients take intranasal insulin or placebo for a year, is set to wrap up in 2018, and ApoE-associated differences will be assessed in that data set.
“This interesting set of findings identifies a new mechanism for how ApoE4 may affect risk for cognitive impairment linked with altering brain insulin signaling,” wrote David Holtzman, Washington University School of Medicine in St. Louis, to Alzforum. “This novel work connects the observations in humans that ApoE4 appears to interact with age and abnormal insulin signaling to increase risk for dementia.”
“This paper shows quite nicely how ApoE4 fails to support insulin signaling and could therefore increase the risk of developing Alzheimer’s by not doing its job well,” said Christian Holscher, Lancaster University, U.K. Holscher is not convinced based on the data presented that ApoE indeed binds directly to the insulin receptor, but agrees with the idea that ApoE4 fails to support insulin signaling relative to ApoE2 or ApoE3.
“We know ApoE is a risk for both diabetes and Alzheimer’s, and this work shows where some causality might come from,” noted Shannon Macauley-Rambach, also at Wake Forest School of Medicine. She wants to know how the proposed mechanism plays out in the context of all the different cell types present in the brain, as well as how this relates back to Aβ and tau, two of the hallmark aggregating proteins in AD.—Gwyneth Dickey Zakaib
References
News Citations
- ApoE Risk Explained? Isoform-Dependent Boost in APP Expression Uncovered
- ApoE Does Not Bind Aβ, Competes for Clearance
Paper Citations
- Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease--is this type 3 diabetes?. J Alzheimers Dis. 2005 Feb;7(1):63-80. PubMed.
- Craft S, Claxton A, Baker LD, Hanson AJ, Cholerton B, Trittschuh EH, Dahl D, Caulder E, Neth B, Montine TJ, Jung Y, Maldjian J, Whitlow C, Friedman S. Effects of Regular and Long-Acting Insulin on Cognition and Alzheimer's Disease Biomarkers: A Pilot Clinical Trial. J Alzheimers Dis. 2017;57(4):1325-1334. PubMed.
Further Reading
Papers
- Traversy MT, Vandal M, Tremblay C, Tournissac M, Giguère-Rancourt A, Bennett AD, Calon F. Altered cerebral insulin response in transgenic mice expressing the epsilon-4 allele of the human apolipoprotein E gene. Psychoneuroendocrinology. 2017 Mar;77:203-210. Epub 2016 Nov 30 PubMed.
- Morris JK, Uy RA, Vidoni ED, Wilkins HM, Archer AE, Thyfault JP, Miles JM, Burns JM. Effect of APOE ε4 Genotype on Metabolic Biomarkers in Aging and Alzheimer's Disease. J Alzheimers Dis. 2017;58(4):1129-1135. PubMed.
- Chan ES, Shetty MS, Sajikumar S, Chen C, Soong TW, Wong BS. ApoE4 expression accelerates hippocampus-dependent cognitive deficits by enhancing Aβ impairment of insulin signaling in an Alzheimer's disease mouse model. Sci Rep. 2016 May 18;6:26119. PubMed.
- Zhao WQ, Lacor PN, Chen H, Lambert MP, Quon MJ, Krafft GA, Klein WL. Insulin receptor dysfunction impairs cellular clearance of neurotoxic oligomeric a{beta}. J Biol Chem. 2009 Jul 10;284(28):18742-53. PubMed.
News
- Do ApoE4 and Diabetes Conspire to Spur Cognitive Decline?
- ApoE Risk Explained? Isoform-Dependent Boost in APP Expression Uncovered
- Aging Mice are Sharper, Fitter on a High-Fat, Low-Carb Diet
- Loss of Tau Function Triggers Insulin Resistance in the Brain
- Put Down That Pop: Sweet Drinks Tied to Stroke, Dementia
- Do ApoE4 and Diabetes Conspire to Spur Cognitive Decline?
Primary Papers
- Zhao N, Liu CC, Van Ingelgom AJ, Martens YA, Linares C, Knight JA, Painter MM, Sullivan PM, Bu G. Apolipoprotein E4 Impairs Neuronal Insulin Signaling by Trapping Insulin Receptor in the Endosomes. Neuron. 2017 Sep 27;96(1):115-129.e5. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
Singapore Institute of Technology
The paper by Zhao et al. and other earlier findings have shown that ApoE4 impairs neuronal insulin signaling. In the current paper, the authors showed both recombinant ApoE3 and ApoE4 can bind to the insulin receptor, with ApoE4 having higher affinity. But when using brain lysates from ApoE targeted replacement (TR) mice, interaction between ApoE4 and insulin receptor was only observed in old, not young, mice. This is similar to our earlier study (Chan et al., 2015) showing no insulin receptor was associated with affinity-captured-ApoE4 (from ApoE TR mice) in the absence of Aβ peptide. This differential association of insulin receptor with ApoE3 and ApoE4 remained with increasing concentrations of Aβ42. In addition, we also observed that the affinity-captured ApoE4 bound more Aβ42 with increasing peptide levels.
However, we observed both ApoE3 and ApoE4 hippocampal neurons (from ApoE TR mice) are equally sensitive to physiological levels of insulin. In the presence of Aβ42, insulin failed to elicit a downstream response only in ApoE4 hippocampal neurons.
Therefore, it will be interesting to examine the effect of Aβ on the entrapment of insulin receptor.
References:
Chan ES, Chen C, Cole GM, Wong BS. Differential interaction of Apolipoprotein-E isoforms with insulin receptors modulates brain insulin signaling in mutant human amyloid precursor protein transgenic mice. Sci Rep. 2015 Sep 8;5:13842. PubMed.
University of California, San Francisco
This new paper on Alzheimer's disease relates pathogenesis to the aging-related, chronically decreasing ability of glucose to cross the blood-brain barrier. The bulk delivery of glucose through the blood-brain barrier is not insulin dependent. A mouse model of AD with ApoE4 showed 29 percent reduced ability of glucose to cross the BBB compared with the ApoE2 model. I believe this helps explain early onset in people carrying this genetic risk factor. The mechanism by which ApoE4 works is essential to understand, but energy deficiency in CNS may be the key. My paper can be found here.
References:
Blonz ER. Alzheimer's Disease as the Product of a Progressive Energy Deficiency Syndrome in the Central Nervous System: The Neuroenergetic Hypothesis. J Alzheimers Dis. 2017;60(4):1223-1229. PubMed.
Wake Forest University School of Medicine
Zhao et al. report an elegant series of experiments that elucidate mechanisms through which ApoE4 can interfere with brain insulin signaling. Their finding that ApoE4 impairs neuronal insulin signaling by binding to the insulin receptor, accelerating its aggregation and thereby preventing its trafficking from endosomal compartments, represents a significant advance in knowledge about mechanisms underlying the effects of ApoE4 and insulin resistance in the brain. They correctly emphasize the potential importance of this finding for designing therapeutic strategies to restore brain insulin function. However, they carry this argument too far by implying that human APOE4 noncarriers with AD do not have brain insulin resistance, based on their results in the APOE3 mice. Talbot et al. (2012) found evidence of brain insulin resistance in the brains of decedent adults with AD regardless of APOE genotype. A more likely scenario is that APOE4 noncarriers with AD have brain insulin resistance caused by a mechanism unrelated to APOE genotype. For example, disturbed lipid metabolism and vascular dysfunction associated with peripheral insulin resistance in APOE4 noncarriers may impact the brain and induce brain insulin resistance. Similarly, Aβ has been shown to induce brain insulin resistance in rodent and nonhuman primate models, so Aβ dysregulation unrelated to APOE4 could induce brain insulin resistance. Consequently, the authors’ speculation that prolonged treatment with intranasal insulin may not benefit APOE4 noncarriers with AD because they have normal brain insulin sensitivity that will be compromised by treatment-induced brain insulin resistance is unfounded. Studies to date have consistently demonstrated benefits of intranasal regular insulin for non-E4 carriers, although they have been relatively short in duration, with a maximum treatment period of six months. An ongoing Phase 2/3 trial will be completed in 2018, in which participants will receive intranasal insulin or placebo for 12 months, with three-month interval assessments. This study will be adequately powered to determine APOE-associated differences in treatment response over this period, and thus provide more definitive clinical data to address this important question.
An additional important question is whether the mechanisms underlying brain insulin resistance in APOE3-associated AD differ from the mechanisms demonstrated for APOE4 in the present paper. Different mechanisms may respond better to certain formulations of insulin or different methods of modulating insulin signaling pathways in the brain, and thus such knowledge would enhance our ability to more precisely target prevention and therapeutic strategies.
References:
Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012 Jan;69(1):29-38. PubMed.
Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012 Apr;122(4):1316-38. PubMed.
Make a Comment
To make a comment you must login or register.