Antisense Oligos Tango with Tau Transcripts to Reverse Tauopathy
Quick Links
Antisense oligonucleotides (ASOs) show promise for treating a host of neurodegenerative disorders, now including tauopathies. In collaboration with the pharmaceutical company Ionis in Carlsbad, California, Timothy Miller and his colleagues at Washington University in St. Louis designed a potent ASO—dubbed TauASO-12—that shuts down human tau gene expression in transgenic mouse models. Compared to controls, animals treated with TauASO-12 had less neurodegeneration, lived longer, and behaved more like normal mice. The researchers also showed that ASOs diffused throughout the brain and spinal cord in nonhuman primates, hinting that injecting them into the spinal fluid might be an effective mode of delivery in people. ASO treatments for other disorders have been packaged into viruses. These findings appeared today in Science Translational Medicine.
“This mouse study is nicely done. It supports the idea that lowering tau levels can be effective against tauopathy,” said Karen Duff of Columbia University, New York. “Because the approach has potential for use in humans, it is particularly exciting.”
Because the toxic effects of tau are concentration-dependent, and previous work has shown that mice tolerate tau reduction, suppressing the protein may benefit people with tauopathies, noted Michel Goedert, Cambridge University, U.K. While people do not overexpress tau as do the mice used in this study, downregulating tau could still be therapeutically beneficial, Goedert said.
Other labs have tried immunotherapy or RNAi to reduce tau. Miller said the jury is out about which approach will work best, but he views tau antisense oligonucleotides as a method with a clear path to the clinic.
Just last month the FDA approved an Ionis ASO—nursinersen—for treatment of spinal muscular atrophy in children and adults. Ionis has ASOs tailored to other genetic neurodegenerative disorders in clinical trials, as well. For example, in August 2015, Ionis started a Phase 1/2 study at six sites in Canada, Germany, and the United Kingdom, in which researchers infuse an ASO directed against expanded huntingtin intrathecally once a month into 36 people with mild Huntington’s disease (see clinicaltrials.gov). Like many sponsors, Ionis insists on monitoring communication from this trial, but a site investigator told Alzforum privately that study recruitment is going well.
Previously, Miller worked with Ionis to develop an ASO that reins in endogenous mouse tau (see Aug 2013 news). TauASO-12 structurally resembles its predecessor and blocks human tau translation in the same way. It comprises a 20 base-pair oligonucleotide complimentary to the P301S variant of human tau that is found in some tauopathies.

Reversing Tau Pathology.
Twelve-month-old PS19 transgenic mice treated with TauASO-12 for a month (bottom) accumulate less phospho-tau in the brain than sham-treated age-matched (middle) or sham-treated nine-month-old mice (top). Green represents AT8 anti-phospho-tau. [Image courtesy of DeVos et al., Science Translational Medicine 2017.]
First author Sarah DeVos tested TauASO-12 in PS19 transgenic mice, which express P301S human tau at about five times the level of normal wild-type mouse tau. At five to six months of age, PS19 mice begin to develop tau deposits. By nine months of age they show extensive neurofibrillary tangles and neuronal cell death. By this age the mice have trouble building nests.
To see whether TauASO-12 could prevent tau pathology, the researchers fitted six-month-old PS19 mice with a tiny osmotic pump that funneled the oligonucleotide directly into the ventricles of the brain at a rate of 30 mg/day. As a control they scrambled the DNA sequence of TauASO-12. Treatment lasted a month, and researchers examined brain tissue two months later. PS19 mice treated with TauASO-12 had about 30 and 60 percent less human tau mRNA and protein, respectively, in the brain. They also developed considerably fewer tau deposits than control animals
Next, the researchers tested the oligonucleotide in a treatment paradigm. DeVos used the same osmotic pump to deliver 30 mg/day TauASO-12 into the brains of nine-month-old PS19 mice for one month, then examined the brains when the animals were 12 months old. The researchers found less human tau mRNA and protein than in the brains of control mice. In fact, the older treated animals had even less tau than the six-month-old mice treated in the prevention paradigm. Treated animals also accumulated much less insoluble and high-molecular-weight forms of the protein. Phosphorylated tau in brain extracts registered barely a blip compared to levels in sham-treated mice. Immunohistochemistry using the AT-8 antibody confirmed this, revealing much less phosphorylated tau in the hippocampi and whole brains than in controls. In fact, treated animals had less AT8 immunoreactivity than even nine-month-old mice, suggesting that the antisense treatment not only prevented new tau pathology, but reversed what was there to begin with (see image above). The results were stark, with little overlap between treatment and controls: The highest AT8 binding in the treatment group was lower than the lowest in both the control group and the nine-month-old sham-treated mice in the prevention paradigm. Miller said this reversal most surprised him. Thioflavin S staining for neurofibrillary tangles supported the idea that treatment reverses pathology. TauASO-12-treated mice had fewer tangles than either 12-month-old sham-treated mice or nine-month-old mice that had been treated in the prevention paradigm.
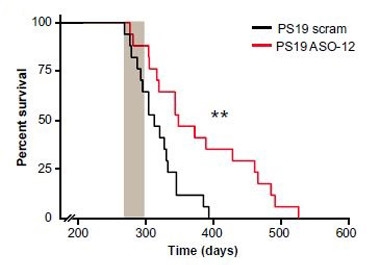
Living Longer.
Tau-overexpressing mice treated with TauASO-12 (red) outlived controls (black). Gray bar shows treatment interval. [Image courtesy of DeVos et al., Science Translational Medicine 2017.]
The reductions in tau pathology seemed to bode well for the mice. DeVos recorded much less inflammation in the brain, as determined by glial fibrillary acidic protein staining. The hippocampus was preserved. P19 mice are known to lose hippocampal volume as they age, but the treated animals had larger hippocampi than controls. NeuN immunoreactivity suggested they retained greater numbers of neurons as well. This could be because the ASO suppressed toxic tau seeds that might travel from cell to cell in the brain, corrupting normal tau in the process. Collaborating with Marc Diamond at the University of Texas Southwestern Medical Center, Dallas, who developed a test for tau seeds when he was at WashU (see Oct 2014 news), DeVos found fewer seeds in brain extracts from treated mouse brains than in extracts from control brains.
What about function? In the treatment paradigm, the PS19 mice lived up to three months longer than those treated with the scrambled ASO. While this works out to an increase in longevity of approximately 25 percent, the animals still lived only half as long as wild-type mice. Nevertheless, one assay hinted they were behaving more normally. One month of TauASO-12 rescued nest-building assessed six weeks later; this is quantified by how much bedding material the mice fail to use, plus a scale that rates the quality of the nests they build (Deacon 2012).
Adam Boxer, University of California San Francisco, cautioned that it’s uncertain if mouse behavioral assays such as nesting predict human clinical responses. Duff said she would like to see a truer test of learning and memory, such as the Morris water maze or fear conditioning, to better understand the effect of tau reduction on cognitive impairment. Overall, researchers have become more skeptical of behavioral testing in mice, questioning how it relates to cognition in people (see Jan 2017 news).
The litmus test will come in human trials. Miller and colleagues plan to test tau ASOs in the clinic. Two immediate challenges are drug delivery and pharmacodynamics. Because they poorly cross the blood-brain barrier, ASOs have to be infused intrathecally into the cerebrospinal fluid or delivered on a genetic vector that easily accesses the brain. Intrathecal delivery has been dogged by the question of how well the drug will perfuse through a large tissue, such as the human brain (see Jan 2013 news). Miller’s group found this route works for TauASO-12 in cynomolgus monkeys. They gave three monkeys 30 mg monkey tau ASO and three other monkeys 50 mg. All were infused via lumbar puncture into the CSF. Two weeks later the scientists harvested tissue across the central nervous system, and documented widespread dose-dependent reduction in tau mRNA.
Next, they infused four monkeys with a bolus of 10 mg tau ASO, followed by another 30mg one week later. After another four weeks they collected the brains and CSF. Compared with baseline levels, tau in the CSF was significantly lower at the time of sacrifice, and the level of tau in the CSF correlated directly with the amount of tau protein in the hippocampus. Boxer considered this result compelling. Finding comparable tau levels in CSF and the hippocampus highlights a biomarker that is easily translatable into the clinic, he suggested. Encouraged by these experiments in animals, Frank Bennett and colleagues at Ionis have been screening for the most potent ASO for the human tau gene. According to Bennett, next up are animal toxicology studies to look for side effects. If all goes well, he expects to begin Phase 1 clinical trials with an optimized tau antisense oligonucleotide even this year.
“There is a clear path for translation through the approach we used in the mouse models and later in the nonhuman primates,” Miller said. “We are enthusiastic about moving this forward.”—Erin Hare
Erin Hare is freelance writer based in Pittsburgh.
References
News Citations
- In Adult Mice, Reduced Tau Quiets Agitated Neurons
- Cellular Biosensor Detects Tau Seeds Long Before They Sprout Pathology
- Building Better Mouse Models for Late-Onset Alzheimer’s
- Cut to the Chase: Therapies Go Directly to Central Nervous System
Mutations Citations
Research Models Citations
Paper Citations
- Deacon R. Assessing burrowing, nest construction, and hoarding in mice. J Vis Exp. 2012;(59):e2607. PubMed.
External Citations
Further Reading
Primary Papers
- DeVos SL, Miller RL, Schoch KM, Holmes BB, Kebodeaux CS, Wegener AJ, Chen G, Shen T, Tran H, Nichols B, Zanardi TA, Kordasiewicz HB, Swayze EE, Bennett CF, Diamond MI, Miller TM. Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci Transl Med. 2017 Jan 25;9(374) PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.