Antibody Ferry Looks Safe in Monkeys, Charts Course for Human Studies
Quick Links
Antibodies hold potential for treating neurodegenerative disease, but these large molecules barely trickle across the blood-brain barrier. Several research groups are working on better delivery methods. One, led by Ryan Watts at Genentech, South San Francisco, California, previously devised a way to sneak antibodies to BACE1 into the brain by hijacking a system the brain uses to transport iron molecules. In wild-type mice, the treatment squelched brain Aβ40 levels, suggesting the antibodies could help slow Alzheimer’s disease. Now, in the 5 November Science Translational Medicine, Watts and colleagues report that the approach works equally well in primates. Moreover, red-blood-cell damage that cropped up in rodent studies did not occur in monkeys, opening the door for human trials.
“This is a very clever, innovative approach,” said Robert Vassar at Northwestern University, Evanston, Illinois. He was not involved in the research. He added that anti-BACE1 antibodies are likely to have fewer side effects than the small-molecule BACE1 inhibitors currently in clinical trials for AD (see Dec 2013 news story; Oct 2014 news story; Oct 2014 news story), because antibodies recognize only a single specific target. “It represents a nice alternative approach. I’m pretty excited about it,” he said.
The bispecific antibody has one arm that recognizes the transferrin receptor and one that binds BACE1.
[Image courtesy of Science Translational Medicine/AAAS.]
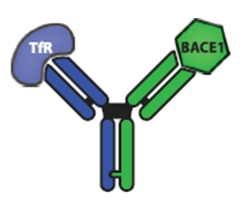
Double-pronged therapy.
In earlier rodent studies, the researchers generated bispecific antibodies in which one arm recognized the mouse transferrin receptor (TfR) expressed by endothelial cells of brain blood vessels, while the other arm targeted BACE1 (see May 2011 news story). To extend the work to humans, joint first authors Joy Yu and Jasvinder Atwal generated antibodies against human TfR, then made bispecific anti-TfR/BACE1 constructs. In mice with a human TfR gene knocked in, a single intravenous dose of 50 mg/kg of the antibody passed easily into the brain, reaching concentrations of about 30 nanomolar and lowering brain Aβ40 levels by about one-third as long as four days later. Monkey TfR varies slightly from the human protein, but a 30 mg/kg dose also performed well in cynomolgus monkeys, reaching concentrations up to 10 nM in the brain and suppressing Aβ40 production by 20 percent for up to 10 days.
The researchers were particularly encouraged by the lack of side effects. In mice, treatment with bispecific antibodies damaged circulating immature red blood cells, which express high levels of TfR (see May 2013 news story). By contrast, treated monkeys maintained normal levels of red blood cells. This is probably because few circulating reticulocytes in primates express the transferrin receptor, the authors claim. Primate blood cells mature in the bone marrow, where they load up on iron and hemoglobin.
Next, the authors will select the final design for a therapeutic molecule to take forward into Phase 1 testing, Watts wrote to Alzforum. In mice and monkeys, antibodies with high affinity to TfR caused degradation of transferrin receptors (see Jan 2014 news story), while those with very low affinity did not penetrate the brain well. The authors will aim for the sweet spot between these two extremes to find a candidate with good brain uptake and sustained effects, Watts wrote.
Other groups are working on similar approaches, including a “brain shuttle” in development at Hoffmann-La Roche, Basel, Switzerland (see Jan 2014 news story). Although Genentech is a member of the Roche Group, the two research programs are independent. In contrast to the natural antibody structure of Genentech’s molecule, Roche’s approach conjoins a single anti-TfR arm to a whole antibody or other drug. In theory, this would allow the brain shuttle to transport several different types of therapeutic by just swapping out the conjugate. The two approaches complement each other, Watts wrote, adding, “We look forward to seeing many unique ways of utilizing TfR to cross the blood-brain barrier.”—Madolyn Bowman Rogers
References
News Citations
- Merck BACE Inhibitor Clears a Safety Hurdle, Gets New Trial
- Lilly Teams Up With AstraZeneca for BACE Inhibitor Phase 2/3 Trial
- Research Brief: New BACE Inhibitor Joins the Fold
- Smuggling Antibodies to BACE Across the Blood-Brain Barrier
- Can Transferrin Antibodies Safely Open the Blood-Brain Barrier?
- Less Is More: High-Affinity Antibodies Block Blood-Brain Barrier Conduit
- Brain Shuttle Ferries Antibodies Across the Blood-Brain Barrier
Further Reading
Primary Papers
- Yu YJ, Atwal JK, Zhang Y, Tong RK, Wildsmith KR, Tan C, Bien-Ly N, Hersom M, Maloney JA, Meilandt WJ, Bumbaca D, Gadkar K, Hoyte K, Luk W, Lu Y, Ernst JA, Scearce-Levie K, Couch JA, Dennis MS, Watts RJ. Therapeutic bispecific antibodies cross the blood-brain barrier in nonhuman primates. Sci Transl Med. 2014 Nov 5;6(261):261ra154. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.