Antibodies Co-Opt Anti-Microbial Response to Clear Intraneuronal Tau
Quick Links
Scientists are developing therapeutic antibodies against pathologic forms of tau, but questions remain about how these antibodies eliminate the protein. While some studies suggest they clear extracellular tau aggregates, a paper in the January 3 Proceedings of the National Academy of Sciences claims they remove intraneuronal tau as well, by recruiting an ancient viral defense mechanism. Researchers led by Michel Goedert and Leo James at the Medical Research Council Laboratory of Molecular Biology, Cambridge, U.K., found that after cultured neuronal cells took up tau aggregates complexed with anti-tau antibodies, the complexes bound to a cytosolic protein, TRIM21. TRIM21 recognizes the Fc region of antibodies and forms part of the cellular defense system against invading microbes. It triggered clearance of the tau particles and prevented them from incorporating cytosolic tau into larger deposits. “The data suggest that cellular antiviral mechanisms could be harnessed to prevent the seeding of toxic tau,” first author William McEwan told Alzforum. That strategy might work for other types of protein aggregate as well, he suggested.
Commenters said the data look solid, although they noted it remains to be seen how much of a role TRIM21 plays in vivo. “This is very interesting work, and helps us understand one facet of how anti-tau antibodies might function,” Marc Diamond at the University of Texas Southwestern Medical Center, Dallas, wrote to Alzforum. Diamond and others stressed, however, that antibodies likely act through multiple mechanisms to stimulate clearance of pathologic tau and other aggregating proteins. “Which pathway is most important depends on the particular antibody and the design of the experiment,” Einar Sigurdsson at the New York School of Medicine told Alzforum.
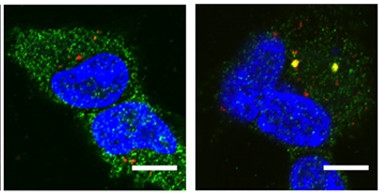
Antibodies Attract.
TRIM21 (green) ignores aggregated tau (red) in cultured HEK293 cells (left), but surrounds tau/anti-tau complexes (yellow, right). Nuclei are blue. [Courtesy of McEwan et al., PNAS.]
James’ lab previously reported that TRIM21, which is found in all cells, helps clear internalized, antibody-bound viruses by triggering their degradation by the proteasome (see Mallery et al., 2010; McEwan et al., 2012). The authors wondered if this mechanism might also help clear misfolded tau, which travels from cell to cell corrupting normal intracellular tau as it goes (see Mar 2009 news; Jun 2009 news).
To test this idea, McEwan and colleagues mixed various anti-tau antibodies with preformed tau assemblies and then added them to a HEK293 cell-based seeding assay they designed for the purpose. The HEK293 cells expressed human mutant P301S tau, which aggregates rapidly when stimulated by tau seeds. Two antibodies, 5A6 and BR134, each cut the fraction of cells that seeded tau aggregation by sevenfold. Neither antibody blocked the cellular uptake of tau, suggesting they acted intracellularly. When the researchers knocked out TRIM21 in the HEK293 cells, however, seeding prowess bounced back fivefold. The authors performed similar experiments in the neuroblastoma cell line SHSY-5Y, also engineered to express human P301S tau, and again found that TRIM21 was essential for antibodies to suppress seeding.
How does TRIM21 work? In the seeding assay, TRIM21 quickly went from floating randomly around in the cytosol to surrounding internalized tau-antibody complexes (see image above). Evidence suggests that this tags complexes for destruction. In the case of viruses, TRIM21 depends on both the proteasome and the chaperone VCP, which unfolds proteins (see Hauler et al., 2012). In the tau seeding assay, inhibiting the proteasome roughly doubled the number of cells that developed tau aggregates. Inhibiting VCP had an even more dramatic effect, nearly abolishing antibody protection. VCP may unfold tau assemblies, thus stopping them from seeding deposits or making them easier to digest, the authors speculated. Knocking down autophagy, on the other hand, had no effect on TRIM21 protection, indicating this process was not involved.
Because SHSY-5Y cells are not identical to neurons, the authors looked for TRIM21 activation in cortical neurons from wild-type mice. They treated them with an adenovirus, with or without the antiviral antibody 9C12. The antibody reduced the number of primary neurons that became infected by 40-fold. In neurons from TRIM21 knockout mice, however, the antibody blocked infection by only 12-fold. The data suggest that TRIM21 does play a key role in protecting neurons—at least from viruses. Treatment with interferon-α and interferon-β boosted TRIM21 expression in wild-type neurons and almost completely suppressed viral infection.
Other researchers called the data compelling. “The results are convincing, providing a detailed mechanism for how antibodies are able to neutralize and eliminate misfolded tau inside cells,” Kun Ping Lu at Harvard Medical School, Boston, wrote to Alzforum. Commenters said the findings have already inspired enthusiastic discussions among researchers in the immunotherapy field, and they suggested the data might help those researchers tweak the design of anti-tau antibodies to improve their effectiveness. All of this hinges, however, on whether TRIM21 also mediates anti-tau antibody protection in the brain. To address that, McEwan and colleagues have crossed TRIM21 knockout mice with animals expressing mutant and wild-type human tau. They will treat the offspring with anti-tau antibodies to find out if mice lacking TRIM21 respond more poorly to this therapy than their intact littermates.
While researchers were excited by the TRIM21 findings, they also pointed out that antibodies can clear protein through several mechanisms, including blocking tau entry and triggering lysosomal degradation inside cells, and that different antibodies are likely to engage different processes (see Jun 2015 news; Feb 2016 news). It remains to be seen how important this TRIM21 route is, they concurred.
One clue to this would be whether other known antibodies act through TRIM21. Lu and colleagues recently reported that TRIM21 activity was crucial for the effectiveness of an anti-tau antibody they studied (see Jul 2015 news). Steven Paul at Weill Cornell Medical College, New York, wonders if TRIM21 might explain recent results from his lab as well. Because systemically administered antibodies poorly penetrate the brain, his group injected an adenoviral vector carrying the anti-tau antibody PHF1 into the hippocampus of P301S tau model mice. This resulted in very high antibody expression in neurons and boosted the effectiveness of the therapy compared to systemic administration. A single injection slashed tau tangles by up to 90 percent three months later (see Liu et al., 2016). Perhaps the intracellular antibodies triggered degradation through TRIM21, Paul speculated. He will explore this in future studies.
Paul believes the findings from the Cambridge group support the use of vectors to achieve high intraneuronal antibody expression. “Their data suggest if you could drive more antibody into the cell, you might have a more effective tau therapy,” Paul told Alzforum. His start-up biotech company, Voyager Therapeutics, will also investigate whether TRIM21 could help clear other types of aggregating protein, such as huntingtin and α-synuclein.
The TRIM21 data also provide an explanation for how “effectorless” antibodies, which lack an Fcγ receptor binding site, might work. Several companies are developing antibodies with reduced effector function, which include crenezumab, to avoid triggering inflammatory microglial signaling and edema (see Jan 2002 news; Oct 2002 news). Some researchers have worried that effector function might be necessary to stimulate microglial phagocytosis and protein clearance, but Gai Ayalon and colleagues at Genentech, South San Francisco, recently reported that RO 7105705, an anti-tau antibody with reduced effector function, mops up tau deposits in mouse hippocampus as well as normal antibodies do (see Lee et al., 2016). McEwan et al.’s findings suggest that one way effectorless antibodies might work is through TRIM21, Ayalon told Alzforum. TRIM21 binds at different a site than the Fcγ receptor does.
What cell types in the brain might most utilize TRIM21? Ayalon wonders if TRIM21 clearance might play an even bigger role in microglia and astrocytes than in neurons. Glial cells express more TRIM21 than neurons do, and are more phagocytic, so they might be more likely than neurons to engulf tau-antibody complexes and degrade them through this mechanism, he speculated. “I wouldn’t be surprised if clearance was predominantly driven by glial cells in the context of the whole brain,” he told Alzforum.
This could be important, as it is unclear how well neurons in vivo would take up tau-antibody complexes. Because neither HEK293 nor SHSY-5Y cells express the Fcγ receptor, these cell types probably took up the complexes by macropinocytosis, a process by which cells engulf bits of their surrounding medium. Neurons have been reported to take up tau in this manner, but Sigurdsson has reported that they internalize only about 20 percent of antibodies in this way, with the remainder brought in by the Fcγ receptor (see Holmes et al., 2013; Congdon et al., 2013). McEwan and colleagues used lipofectamine in their cell culture experiments to permeabilize cells and stimulate uptake, which would not be an option in vivo.—Madolyn Bowman Rogers
References
News Citations
- Double Paper Alert—Keystone Presentations Now in Press
- Traveling Tau—A New Paradigm for Tau- and Other Proteinopathies?
- Antibodies Boost Microglial Appetite for Tau
- Bon Appétit: Endogenous Antibodies Prod Microglia to Eat Aβ Deposits
- Could Kink in Tau Lead to Neurodegeneration?
- Human Aβ Vaccine Snagged by CNS Inflammation
- The Alzheimer's Vaccination Story, Continued
Research Models Citations
Therapeutics Citations
Paper Citations
- Mallery DL, McEwan WA, Bidgood SR, Towers GJ, Johnson CM, James LC. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21). Proc Natl Acad Sci U S A. 2010 Nov 16;107(46):19985-90. Epub 2010 Nov 2 PubMed.
- McEwan WA, Hauler F, Williams CR, Bidgood SR, Mallery DL, Crowther RA, James LC. Regulation of virus neutralization and the persistent fraction by TRIM21. J Virol. 2012 Aug;86(16):8482-91. Epub 2012 May 30 PubMed.
- Hauler F, Mallery DL, McEwan WA, Bidgood SR, James LC. AAA ATPase p97/VCP is essential for TRIM21-mediated virus neutralization. Proc Natl Acad Sci U S A. 2012 Nov 27;109(48):19733-8. Epub 2012 Oct 22 PubMed.
- Liu W, Zhao L, Blackman B, Parmar M, Wong MY, Woo T, Yu F, Chiuchiolo MJ, Sondhi D, Kaminsky SM, Crystal RG, Paul SM. Vectored Intracerebral Immunization with the Anti-Tau Monoclonal Antibody PHF1 Markedly Reduces Tau Pathology in Mutant Tau Transgenic Mice. J Neurosci. 2016 Dec 7;36(49):12425-12435. PubMed.
- Lee SH, Le Pichon CE, Adolfsson O, Gafner V, Pihlgren M, Lin H, Solanoy H, Brendza R, Ngu H, Foreman O, Chan R, Ernst JA, DiCara D, Hotzel I, Srinivasan K, Hansen DV, Atwal J, Lu Y, Bumbaca D, Pfeifer A, Watts RJ, Muhs A, Scearce-Levie K, Ayalon G. Antibody-Mediated Targeting of Tau In Vivo Does Not Require Effector Function and Microglial Engagement. Cell Rep. 2016 Aug 9;16(6):1690-700. Epub 2016 Jul 28 PubMed.
- Holmes BB, DeVos SL, Kfoury N, Li M, Jacks R, Yanamandra K, Ouidja MO, Brodsky FM, Marasa J, Bagchi DP, Kotzbauer PT, Miller TM, Papy-Garcia D, Diamond MI. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc Natl Acad Sci U S A. 2013 Aug 13;110(33):E3138-47. Epub 2013 Jul 29 PubMed.
- Congdon EE, Gu J, Sait HB, Sigurdsson EM. Antibody uptake into neurons occurs primarily via clathrin-dependent Fcγ receptor endocytosis and is a prerequisite for acute tau protein clearance. J Biol Chem. 2013 Dec 6;288(49):35452-65. Epub 2013 Oct 25 PubMed.
Further Reading
Primary Papers
- McEwan WA, Falcon B, Vaysburd M, Clift D, Oblak AL, Ghetti B, Goedert M, James LC. Cytosolic Fc receptor TRIM21 inhibits seeded tau aggregation. Proc Natl Acad Sci U S A. 2017 Jan 17;114(3):574-579. Epub 2017 Jan 3 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.