Aha! Co-Conspirator Caught Misfolding Tau
Quick Links
Tau misfolds and clumps up in various tauopathies, including Alzheimer’s disease. The chaperone heat shock protein 90 (Hsp90) helps create those irregular crinkles, but scientists have met little success in trying to block this chaperone therapeutically. Could they have found the next best thing? A co-chaperone called Aha1 speeds up the twisting of tau, report researchers at the University of South Florida, Tampa, who were led by the late Chad Dickey (Nov 2016 news). In the August 21 PNAS, they describe how blocking Aha1 slows tau fibril formation in vitro and how the co-chaperone co-localizes with tangles in the human brain, supporting the notion that it contributes to pathology. They go on to show that tau transgenic mice overexpressing Aha1 develop more tangles and worse memory impairments than controls. “This is an impressive study,” said Markus Zweckstetter, Max Planck Institute for Biophysical Chemistry in Göttingen, Germany. “It goes from in vitro experiments all the way to animal models and patient materials; the broad scope is very nice.”
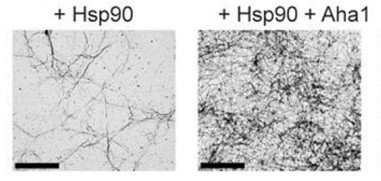
Tangling tau.
On its own, Hsp90 causes some fibrils to form (left). Aha1 (right), exacerbates the process. [Courtesy of Lindsey Shelton, PNAS.]
Hsp90 hunts down free-floating misfolded proteins. When it finds misfolded tau, it can shunt it in one of two ways depending on the co-chaperones recruited. If CHIP hooks on, the complex sends tau for degradation. If FKBP51 binds Hsp90 instead, the complex tries to refold tau correctly but fails, crumpling it into an aggregating conformation (Sulistio and Heese, 2015). Researchers thought until recently that reducing Hsp90 activity would reduce tau aggregation, but inhibitors failed to cross into the brain well and proved toxic (Butler et al., 2015). Scientists are now going after the co-chaperones that aid and abet Hsp90, hoping they may be better drug targets.
First author Lindsey Shelton and colleagues tested five known co-chaperones of Hsp90 to see if any hastened or slowed tau aggregation. According to transmission electron microscopy, Aha1 and Hsp90 sped up aggregation of P301L tau relative to Hsp90 alone (see image above). Aha1 is the only partner known to stimulate the chaperone’s ATPase activity, meaning it speeds up Hsp90’s action.
To see if dialing back Aha1 activity could help reduce tau fibrils, the group developed an inhibitor called KU-177. It prevents Aha1 from forming a complex with Hsp90. Since it does not bind to the latter, it should not be as toxic as Hsp90 inhibitors, the authors wrote. KU-177 almost halved the number of fibrils that formed when Shelton mixed P301L tau with Hsp90 and Aha1.
Doubtful that KU-177 can cross into the brain, Blair and colleagues did not test the inhibitor in animals. The lab continues to work with medicinal chemist and co-author Brian Blagg, University of Notre Dame, Indiana, whose lab generated KU-177, to develop derivatives with better drug properties.
What about crossing Aha1 knockout mice with tau models? Researchers led by Didier Picard at the University of Geneva have generated Aha1 knockouts, and Picard told Alzforum these are viable with no obvious phenotype. However, they are still characterizing the mice and have not yet shared them with other labs for technical reasons, though Picard said in theory they are available.
In the meantime, Blair took the opposite approach, asking if overexpression of Aha1 encourages tau to accumulate. They injected AAV viral constructs containing the Aha1 gene into the hippocampi of five month-old rTg4510 mice, which overexpress human P301L tau, and looked for tau accumulation two months later. The infected animals ended up with more insoluble tau and more toxic oligomers than did mock transfected controls. Not only did the injections kill off hippocampal CA1 neurons (see image below), but the mice had trouble finding a hidden platform in the two-dimensional radial arm water maze, suggesting memory deficits.
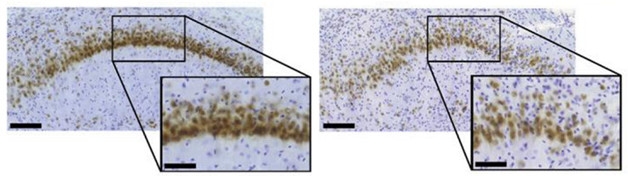
Losing Neurons. Neurons (brown) are sparse in the CA1 region of mice injected with AAV-Aha1 (right) compared to CA1 neurons from mice injected with a control virus (left). [Courtesy of Lindsey Shelton, PNAS.]
The final piece of evidence for Aha1’s co-conspirator status came from human tissue. In postmortem brain samples from AD patients, antibodies to Aha1 co-localized with tau tangles. The staining intensified with increasing Braak stage (see image below).

Aha1 Gets Tangled.
Aha1 (red) co-localization with tau tangles (green) intensified between Braak stages 5 (left) and 6 (right). [Courtesy of Lindsey Shelton, PNAS.]
Davide Tampellini, Inserm, Université Paris Sud, thought that identifying Aha1 as a novel target that might prevent pathological tau species is important for AD and other Hsp90-related disorders. “The main challenge that will need to be addressed is whether KU-177 will work in vivo,” he wrote.—Gwyneth Dickey Zakaib
References
News Citations
Research Models Citations
Paper Citations
- Sulistio YA, Heese K. The Ubiquitin-Proteasome System and Molecular Chaperone Deregulation in Alzheimer's Disease. Mol Neurobiol. 2015 Jan 7; PubMed.
- Butler LM, Ferraldeschi R, Armstrong HK, Centenera MM, Workman P. Maximizing the Therapeutic Potential of HSP90 Inhibitors. Mol Cancer Res. 2015 Nov;13(11):1445-51. Epub 2015 Jul 28 PubMed.
Further Reading
Papers
- Oroz J, Kim JH, Chang BJ, Zweckstetter M. Mechanistic basis for the recognition of a misfolded protein by the molecular chaperone Hsp90. Nat Struct Mol Biol. 2017 Apr;24(4):407-413. Epub 2017 Feb 20 PubMed.
- Blair LJ, Sabbagh JJ, Dickey CA. Targeting Hsp90 and its co-chaperones to treat Alzheimer's disease. Expert Opin Ther Targets. 2014 Oct;18(10):1219-32. Epub 2014 Jul 29 PubMed.
Primary Papers
- Shelton LB, Baker JD, Zheng D, Sullivan LE, Solanki PK, Webster JM, Sun Z, Sabbagh JJ, Nordhues BA, Koren J 3rd, Ghosh S, Blagg BS, Blair LJ, Dickey CA. Hsp90 activator Aha1 drives production of pathological tau aggregates. Proc Natl Acad Sci U S A. 2017 Sep 5;114(36):9707-9712. Epub 2017 Aug 21 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.