VasCog 2015—Highlights of a Conference
Quick Links
This meeting report was contributed to Alzforum by Donna Wilcock, University of Kentucky, Lexington.
The International Society of Vascular Behavioral and Cognitive Disorders, aka VasCog, held its annual meeting in the beautiful city of Tokyo from September 16 to 19. Co-organized by Koji Abe of Okayama University, Japan, and Ken Nagata of the Research Institute for Brain and Blood Vessels, Akita, Japan, this conference spotlighted work being done in Japan and internationally to advance the understanding of vascular contributions to cognitive impairment and dementia (VCID). Many presentations focused on better diagnosis of post-stroke dementia, characterization of the dementia trajectories, and risk factor characterization for VCID. While a brief report cannot highlight all the work presented at this conference, some themes stood out and are highlighted below.
Scientific sessions opened with Sandra Black of Sunnybrook Research Institute, Toronto, who presented data on the venular network in the brain. Black reported that periventricular white-matter hyperintensities (WMH) are prevalent in the elderly. Given the density of veins close to the periventricular spaces, she hypothesizes that thickening venule walls in this region contribute significantly to the presence of WMHs. Thickening walls will also disrupt the periventricular clearance of solutes, such as Aβ. Along the lines of clearance, Roxana Carare of Southampton University, England, and her Ph.D. student Alan William Joe Morris reviewed the perivascular drainage pathways in the brain and potential mechanisms by which the process can be disrupted, leading to Aβ accumulation in the brain parenchyma and vessels. Aβ is known to accumulate along basement membranes of arteries, in between circular layers of smooth muscle cells. In addition, loss of perivascular cholinergic innervation appears to disrupt solute drainage, as does high fat diet administration in adult mice. Carare suggested that increasing perivascular drainage may have therapeutic potential.
Many presentations dealt with diagnosis and trajectory of post-stroke dementia. Olivier Godefroy of Amiens University Hospital, Salouel, France, investigated the prevalence of post-stroke dementia in hospitalized cohorts. By performing a meta-analysis, he found a 53.8 percent prevalence of post-stroke cognitive impairment across 11 cohorts; the post-stroke assessment period was between six months and two years depending on the individual study. Bonnie Yin Ka Lam of the University of Hong Kong studies differences between early onset (within a few months) and late-onset (many months later) post-stroke dementia. Presenting data on ApoE, with some PiB PET imaging, she reported that ApoE4 status was not associated with who develops dementia, and PiB retention was the same between people developing dementia and those who remained cognitively normal.
Koji Abe of Okayama University presented several talks describing the prevalence of white-matter hyperintensities and of dementia in stroke populations and elderly people with mild cognitive impairment. Abe showed that prevalence of WMH is age-dependent, with many over the age of 80 showing WMH. In people with post-stroke dementia, WMH are particularly striking and significantly increased, Abe said. Finally, WMH are more prevalent in individuals presenting with mild cognitive impairment.
Louise Marie Allen of Newcastle University in England presented on 355 stroke survivors in the Newcastle CogFAST (Cognitive Function After Stroke) study. Of these participants, 25 percent had some form of cognitive impairment without dementia three months after their stroke. By the first annual follow up, 13.7 percent of this group still had cognitive impairment without dementia, 9.6 percent had progressed to dementia, and the remainder had recovered. Over the first five-year follow up, the percentage with any form of cognitive impairment remained stable, however, transition from cognitive impairment without dementia to full dementia continued to increase year to year. Additionally, Allen noted that over the 10-year follow up, many participants transitioned between states of impairment, indicating that vascular cognitive impairment is variable and fluid.
XinXin Guo of the University of Gothenburg, Sweden, examined a population study in 1,460 women described as in mid- to late-life at enrollment, without stroke or dementia at baseline, who were followed for 38 years. Of these, 321 women developed stroke or transient ischemic attacks (TIA) and 244 developed dementia. The cumulative incidence of dementia was significantly higher in women who had had a stroke or TIA than in those without, even when adjusted for age. Conversely, as well, the risk of suffering a first stroke or TIA was increased in women who had dementia, even age-adjusted. These data indicate a bidirectional relationship between stroke and dementia over a lifetime.
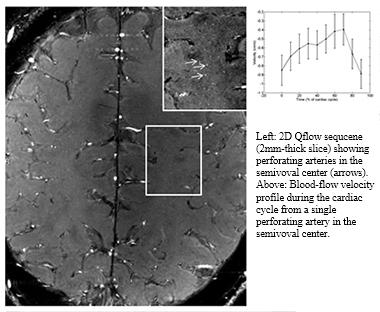
7T MRI of cerebral white matter of a healthy volunteer. Arrows point to perforating arteries; they are 100 microns across. Insert indicates how fast blood flows through one such artery during time of a single, complete heartbeat. [Courtesy Willem Bouvy and Jaco Zwanenburg, University Medical Centre Utrecht.]
A highlight of this meeting was a debate on the question of whether vascular risk factors make a major contribution to the etiology of Alzheimer’s disease. The conversation centered around the incidence of Alzheimer’s in people with risk factors such as stroke, hypertension, obesity, and high BMI, as well as cholesterol and lipid profiles. The data strongly supports an increased risk of Alzheimer’s disease when vascular risk factors are present. However, etiology by definition indicates cause, and the data is merely correlative at this point. Furthermore, the influence of vascular risk factors may be affected by whether the Alzheimer’s diagnosis has been made clinically or neuropathologically, since the vascular factors could likely be leading to vascular cognitive impairment and not the plaques and tangles that define Alzheimer’s disease. Therefore, the panel rephrased the question to, “Do vascular risk factors make a major contribution to the clinical syndrome of dementia?” Addressing the question in its original form would require longitudinal tracking of both vascular and AD biomarkers in aging cohorts and pathological confirmation of lifetime diagnoses.
Characterizing vascular pathologies that are contributing to vascular cognitive impairment and dementia remains a challenge for the field. It is being addressed on several fronts, from imaging to neuropathology and preclinical work in animal models. Geert Jan Biessels of the University Medical Center, Utrecht, Netherlands, described small-vessel disease as characterized by 7T MRI imaging. Biessels showed that he now has methods to examine tortuosity of vessels and perivascular spaces. This brings MRI to bear on the assessment of vascular changes in living people that have previously only been observed in animal models or postmortem brain samples. Further, Biessels showed that using 2D Qflow technology on the 7T MRI enables measurement of single-beat blood flow in small vessels. This could become a readout for vascular stiffness in small-vessel disease, Biessels said.
In his presentation characterizing small-vessel disease, Matthijs Biesbroek, also of University Medical Center, focused on WMH as detected by MRI in a cohort of 167 patients with small-vessel disease. The group found that strategically localized WMHs impaired executive functioning and visuomotor speed; moreover, WMHs associated with specific white-matter tracts appear to affect these cognitive functions strongly.
Aiqing Chen of Newcastle University presented neuropathological data examining astrocytes in post-stroke brains. Clasmatodendrosis, a condition of irreversible damage of perivascular astrocytes, was a significant characteristic of the post-stroke dementia group as compared to the post-stroke non-dementia group. This data strengthens the hypothesis that astrocyte damage at the neurovascular unit is an important mechanism by which cerebrovascular disease contributes to cognitive impairment.

Progressive degeneration of astrocytes in post-stroke dementia. Deep white matter stained for GFAP (green), aquaporin 4 (red), nuclei (blue). In post-stroke patient without dementia (A), AQP4 is mainly on astrocyte end-feet, frequently outlining a vessel. B-D: A patient with post-stroke dementia. B: Astrocyte with intermediate level of pathology. AQP4 seen along cellular process and in cellular membrane (arrow). C: AQP4 seen aggregated in peripheral deposits along swollen astrocyte. D: Collapsed astrocytes with fragmented processes show AQP4 along edge of cell bodies (arrows). Magnification bars, 20µm. [Courtesy of Aiqing Chen, University of Newcastle.]
Several presentations directly addressed the comorbidity of vascular cognitive impairment and Alzheimer’s disease. William Jagust of the University of California, Berkeley, discussed the relationships between brain amyloid burden, cerebrovascular disease and cognition. Using the Framingham Cardiovascular Risk Profile (FCRP), Jagust showed that the higher a person’s FCRP, the higher his or her risk of having amyloid deposition in the brain by PET imaging.
Susanne J. van Veluw of the University Medical Center in Utrecht presented MRI studies of five cases with pathologically confirmed cerebral amyloid angiopathy, in which the autopsied brain was imaged. These CAA brains show increased dilation of the perivascular spaces. Importantly, while increased CAA correlated to the degree of perivascular space dilation, parenchymal amyloid load did not. Jessica Duncombe of the University of Edinburgh presented a study in the TgSwDI mouse model of CAA. By modeling chronic cerebral hypoperfusion through microcoil application on the common carotid arteries of this model, the group showed that brain amyloid load increased significantly. Christian Bocti at the University of Sherbrooke, Quebec, presented data showing that white-matter hyperintensities and brain amyloid load as measured by PiB PET have additive effects in a normal cognitive aging cohort of 76 healthy older adults (mean age of 73).
In sum, these data strongly point to a vascular contribution to cognitive impairment and dementia, but it is variable in both its pathological presentation and the clinical cognitive domains affected. The co-occurrence of cerebrovascular and Alzheimer’s disease is a major challenge in the dementia field at present, but combined clinical and basic science is advancing the study of vascular-neurodegenerative interactions.
This review is not comprehensive, but highlights aspects of the meeting. Other speakers are invited to fill in the picture, and add points of discussion. The next VasCog meeting will be held in Amsterdam from October 12 to October 15, 2016. Alzforum readers are welcome to join.—Donna Wilcock
References
Research Models Citations
Conference Citations
Other Citations
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.