Toward FTD Therapeutic Trials: Diagnosis Firm, Outcomes Still Soft
Quick Links
On 4 June in Washington, D.C., scientists from academia, industry, and funding organizations met to discuss precompetitive steps in the preparation for clinical trials for frontotemporal degeneration. Frontotemporal degeneration (FTD) encompasses a group of diseases that are distinct from Alzheimer’s, the most common neurodegenerative disease. At the same time, FTD exists on a broad spectrum with AD, and a partial overlap in symptoms frequently leads to misdiagnosis of one for the other. Part 2 of this series summarizes components of future trials where clinician-researchers have reached broad consensus—that is, its subtypes and diagnosis—as well as others where more work is needed—that is, endpoints to measure the success or failure of a drug.
Frontotemporal degeneration was first recognized by the Czech neurologist and psychiatrist Arnold Pick, who described it as early as 1892 as a focal neurodegenerative disorder. “That was incredibly modern at the time,” said Bruce Miller of University of California, San Francisco. In 1957, French scientists published a detailed comparison of FTLD and AD (Delay et al., 1957; discussed in Thibodeau and Miller, 2012). Today, scientists of the FTD Treatment Study Group (FTSG) see FTLD as falling into three major groups. The behavioral variant (bvFTD) is a social disorder that starts with psychiatric symptoms, whereas two language variants called progressive aphasia are marked either by difficulties with semantic understanding (svPPA) or by fluency and grammar (nfvPPA/agrammatic PPA). FTLD is rare enough to qualify for orphan disease status (see more in Part 4 of this series), but is actually as common as AD among people age 45 to 64, when FTD strikes primarily.
A large fraction of FTLD cases are diseases of tau. This includes rare genetic forms such as FTDP-17, as well as sporadic conditions that have overlapping symptoms with movement disorders, i.e., progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD). More recently, tauopathies broadly have further come to include an anticipated wave of cases of chronic traumatic encephalopathy (CTE) generated by concussive or blast injuries in contact sports and war (see ARF related news story). “I think we will experience an epidemic related to tau,” Miller told the audience in D.C.
Other large fractions of the FTD spectrum are accounted for by the C9ORF72 and progranulin genes and by TDP-43 pathology; a small slice is attributable to the FUS and CHMP2B genes. For details on the current classification of FTD, see image.
In daily medical practice, particularly in primary care, FTD and AD can easily be misdiagnosed. Typically, people get the better-known AD diagnosis when, in fact, they have a form of FTD. While both diseases impair a person’s executive function, FTD erodes a person’s social and interpersonal behavior but spares memory and visuospatial function, which deteriorate in AD. Importantly, both FTD and AD now have new consensus sets of diagnostic criteria that emphasize the value of biomarkers, said Bradford Dickerson of Massachusetts General Hospital, who runs the largest FTLD research program in New England. At the AFTD conference, representatives of the Food and Drug Administration signaled that they see no problem with using these criteria for grouping patients in clinical trials (see Part 4 of this series). With this, one obstacle for good trials—sorting the right patients into the right trials and treatment groups—appears surmountable.
The subtypes of FTLD are sufficiently well understood to begin grouping them for trials by drug type, Dickerson said. For example, tau-based drugs could be tested in bvFTD, agrammatic PPA, PSP, and CBD, while people with semantic PPA or the motor neuron disease variant of FTD should be excluded. These last two forms of FTLD would be candidates for trials of drugs targeting progranulin or TDP-43.
For bvFTD, consensus clinical criteria (Raskovsky et al., 2011), together with a finding of frontal lobe atrophy on MRI or perhaps a negative amyloid PET scan, can render a diagnosis with great confidence, said Dickerson. The same is true for FTD’s language variants. There, new consensus diagnostic criteria describe the characteristics of the three clinical subtypes of PPA (Gorno-Tempini et al., 2011), and structural MRI reveals a stereotypical pattern of asymmetric frontoinsular atrophy that corresponds to the language symptoms. In a collaboration between Dickerson and Marsel Mesulam at Northwestern University, Chicago, atrophy patterns were consistent between patients seen at MGH and Northwestern. Longitudinally, too, independent MRI studies at different centers are generating data on atrophy rates that agree with each other and begin to support sample size calculations for trials, said Howard Rosen of UCSF. Overall, atrophy rates are nearly twice as fast in FTD as in AD, said Rosen.
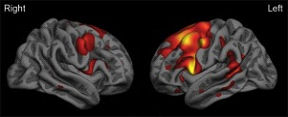
Surface map of the cerebral cortex from a group of patients with the agrammatic variant of primary progressive aphasia demonstrating left-greater-than-right frontal lobe atrophy (red-orange-yellow indicate areas of thinner cortex in patients versus similarly aged controls). Image courtesy of Jason Warren

Surface map of the cerebral cortex from a group of patients with the semantic variant of primary progressive aphasia demonstrating left-greater-than-right anterior temporal lobe atrophy (red-orange-yellow indicate areas of thinner cortex in patients versus similarly aged controls). Image courtesy of Jason Warren
Still, biomarkers are necessary to reduce diagnostic errors in multicenter trials, said Murray Grossman of the University of Pennsylvania in Philadelphia. On the imaging front, tau PET is desirable, as it specifies an underlying molecular pathology, which FDG-PET and MRI cannot do. However, PET is only just entering the first human studies (see 2012 ARF HAI conference report and Zhang et al., 2012), whereas the last two are already widely available. With PET, one concern beyond cost and radiation exposure is that it requires longer scanning times. Some FTLD patients simply do not lie still long enough to conduct a PET scan plus the MRI scan necessary for co-registration, scientists at cautioned in D.C. Partly for this reason, functional connectivity MRI is rising as an experimental modality in the FTLD field. For its part, structural MRI will soon become more specific with multimodal imaging combining gray and white matter, Grossman said.
All told, the general consensus of the day was that structural MRI, while not perfect, is advanced enough for use in therapeutic studies. “The clinical and imaging measures work for trials,” said Dickerson (see Sapolsky et al., 2010). Initial learning trials will precede subsequent registration trials, industry scientists cautioned.
While FTLD research lags behind AD research in establishing positive biomarkers for diagnosis, it benefits nonetheless from those successes in AD. In particular, amyloid PET scans will be a boon for FTLD trials, researchers agreed. “With amyloid imaging we can now predict with almost 100 percent certainty who is on an FTLD path and who is on an AD path. This is helping us tremendously to diagnose FTLD,” said Miller. Dickerson put it this way: “The approval of amyloid PET is going to revolutionize clinical trials. According to the FDA, the main indication is in identifying people who are negative for amyloid. That will expose more FTLD cases.” Ironically, perhaps, a milestone in AD research will raise the profile of other insidious diseases that have long languished in its shadow.
Progress is less clear on fluid measures. In blood, genetic markers for some forms of FTLD are both sensitive and specific. With 30 percent of cases having a family history, blood tests can capture a fair number of patients. The progranulin gene alone is thought to account for up to 10 percent of all cases of FTD. At the same time, the majority of cases are sporadic, and neither genetics nor proteomic studies have yet provided robust fluid markers for them. “We need large-scale proteomic markers,” said Grossman. CSF AD research is helpful in the same indirect way as amyloid imaging, in that the Alzheimer’s Aβ42/tau signature distinguishes FTLD from AD. However, CSF tau at present is curiously uninformative for tau forms of FTLD. In those, even in pure tauopathies, patients have the same levels as controls, perhaps because the assay does not capture the particular tau species altered in those diseases. A robust TDP-43 assay that works reliably in multiple centers is not yet available, and data on CSF progranulin remain sparse.
Once patients are enrolled into a therapeutic study, how will the trial measure success or failure of the drug? The day saw extensive discussion, but little consensus, about the quality of outcome measures in FTLD. In general, outcome measures for FTLD trials were seen as less solid than diagnostic criteria for inclusion/exclusion. While FTLD strikes younger, otherwise healthy people and progresses faster than AD, it is also more clinically heterogeneous, and this poses striking challenges for measuring outcomes. But the issue is solvable, said Howard Feldman of the University of British Columbia, Vancouver, Canada. “We struggle with how we look for drug effect, but it occurs to me that if people fall apart over one to two years, we should be able to capture that.”
To do that, different groups use different clinical rating scales. Some adapt tools for AD, such as multi-domain cognitive composites co-developed by David Knopman of the Mayo Clinic in Rochester, Minnesota, or an FTLD-specific version of the Clinical Dementia Rating Scale (CDRS), an established scale that measures behavior and everyday function. Both form part of an FTLD module recently added to National Alzheimer’s Coordinating Center (NACC) assessments (see ARF related news story). Other groups use their own ratings, such as the Addenbrooke’s Cognitive Examination-revised (ACE-R.) In short, the different domains affected in the different variants of FTD—cognitive, executive, psychiatric, global—have been examined with different tests and partially validated in different longitudinal multicenter studies. For measuring activities of daily living, the Functional Activities Questionnaire (FAQ) seemed acceptable to the assembled scientists. No clear frontrunner for use in treatment trials has emerged; however, the ACE-R has published reports of longitudinal sensitivity as well as translation into major world languages other than English to its credit. The standard 6-domain CDR has also been translated, but the newer FTLD-specific CRD as-yet has not.
The group discussed whether the Frontotemporal Temporal Dementia Study Group (FTSG) should develop its own "perfect" scales or use existing scales. Petra Kaufman of the NINDS offered this advice: “Do not make the perfect the enemy of the good. There can be huge effort in improving an outcome measure, and then, in the end, a very simple measure is eventually chosen.” This is true especially for international studies.
There was agreement that, in general, cognitive/functional scales may work better in trials than behavioral/psychiatric ones. For the neuropsychiatric inventory (NPI), in particular, scientists cautioned that patients falsely appear to improve on it as their disease progresses toward increasing apathy. They do "better" on the scale because they withdraw socially and commit fewer of the inappropriate acts (e.g., touching people, reckless spending) that the scale also captures. Essentially, the NPI captures positive and negative symptoms that can cancel each other out. Such scales are sensitive over the short term to measure a bump in symptomatic improvement, but likely not in long trials needed to show disease modification.
In FTD “long,” as in “needed for Phase 3,” will mean 12 months, not the 18 or even 24 months customary in AD, Knopman suggested. Likewise, Phase 2 trials ought to last, at the utmost, six months. That is because the disease progresses so quickly that many participants won’t stay within the dynamic range of assessments, or even be able to perform study tasks much past one year. When participants leave a trial prematurely, it loses statistical power.
What does all this amount to for therapeutic studies? “We must be honest that with the outcome measures we have, we will need fairly large trials. Because of FTD’s rarity, this may mean international trials; hence, the outcome measures will be administered in local languages and should be simple. But trials are definitely feasible,” Knopman said.—Gabrielle Strobel.
This is Part 2 of a five-part series. See also Part 1, Part 3, Part 4, Part 5. Read a PDF of the entire series.
References
News Citations
- Take It From the Agency: First FTD Drug May Be an Orphan
- Blast Anatomy—Chronic Traumatic Encephalopathy in Military Vets
- Miami: Scientists Angle for Way to Image Tangle
- Scientists Strategize With Regulators for Frontal Assault on FTD
- Case Studies Crystallize Trial Ideas at FTD Conference
- For FTD Drug Development, a Q&A With Regulators
Paper Citations
- DELAY J, BRION S, ESCOUROLLE R. [Anatomico-clinical comparison of Pick's disease and Alzheimer's disease; study of 38 cases]. Presse Med. 1957 Sep 21;65(67):1495-7 contd. PubMed.
- Thibodeau MP, Miller BL. 'Limits and current knowledge of Pick's disease: its differential diagnosis' A translation of the 1957 Delay, Brion, Escourolle article. Neurocase. 2012 May 4; PubMed.
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011 Sep;134(Pt 9):2456-77. Epub 2011 Aug 2 PubMed.
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011 Mar 15;76(11):1006-14. PubMed.
- Zhang W, Arteaga J, Cashion DK, Chen G, Gangadharmath U, Gomez LF, Kasi D, Lam C, Liang Q, Liu C, Mocharla VP, Mu F, Sinha A, Szardenings AK, Wang E, Walsh JC, Xia C, Yu C, Zhao T, Kolb HC. A Highly Selective and Specific PET Tracer for Imaging of Tau Pathologies. J Alzheimers Dis. 2012 Jan 1;31(3):601-12. PubMed.
Other Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.