Tau PET in Down’s: Unique Patterns Among Alzheimer’s Types and Stages
Quick Links
By the time memory frays, the brains of people with Alzheimer’s disease are already infested with Aβ plaques, but the growth of tau tangles tracks with worsening symptoms. With tau PET, researchers now have a tool to watch this happen, and at the Clinical Trials on Alzheimer’s Disease (CTAD) conference, held December 8-10 in San Diego, they discussed their latest data to see how close the field is to using tau PET in human therapy studies. Showing where tau tangles are in people with early versus late-onset AD, Down’s syndrome (DS), and among participants in secondary prevention trials, they compared the differences in tangle distribution. The extent to which tau pathology pushed into the neocortex differed among forms of the disease, but the requirement that Aβ pathology be there to unleash tau’s toxic spread was consistent between studies. Researchers grappled with how best to wield this powerful imaging tool in clinical trials.
A new kid on the block compared to amyloid-PET imaging, tau PET has allowed researchers to confirm during life Braak’s postmortem neuropathology finding that tangle pathology spreads out of the medial temporal lobe into the neocortex as AD progresses (see Braak and Braak, 1991). When combined with the more established amyloid-PET or measurements of CSF Aβ, tau imaging has also implicated elevated Aβ deposition as a prerequisite for tau’s pathological spread into the neocortex (see Mar 2016 news; May 2016 news). Trialists hope to harness this biomarker to select participants and perhaps measure outcomes, but CTAD showed they still have a long way to go before they can lean on tau imaging with confidence. “It’s still early days,” said Michael Schöll of Lund and Gothenberg universities in Sweden.
At CTAD, Reisa Sperling of Brigham and Women’s Hospital in Boston presented the first tau PET data from A4, the ongoing secondary prevention trial testing solanezumab in cognitively normal people with elevated Aβ deposition (see Jan 2013 news). Thus far, nearly 250 tau PET scans have been performed on a subset of the more than 800 participants already randomized across 67 sites in the United States, Canada, Australia, and Japan, Sperling said. While Sperling stressed that no consensus yet exists for a “tau-positive” threshold, she reported that 56 percent of the cohort had standardized uptake volume ratios (SUVRs) of the tau tracer AV-1451 above 1.2, which she classified as elevated neocortical tau.
Sperling reported that even within this presymptomatic cohort, the toxic relationship between Aβ and tau appeared to be firmly established. People at the high end of the Aβ spectrum had more tau pathology in the entorhinal, inferior temporal, and inferior parietal cortices, and precuneus. Sperling told Alzforum that, to her surprise, tau accumulation among A4 participants ranged widely, from primarily medial temporal tangles in some participants to extensive neocortical pathology on par with later Braak stages in others. Sperling referred to people at the high end of the tau spectrum as possible “bell-ringers,” people whose PET data is loudly signaling that they are on the edge of cognitive decline. She said that tau imaging in this cohort may help researchers home in on an ideal treatment window for trials—a time just prior to clinical expression of the underlying disease process—and also to investigate how Aβ-targeted therapies alter the course of tau pathology.
Michael Rafii of the Alzheimer’s Therapeutic Research Institute at the University of Southern California and the University of California, San Diego, presented data from the first tau PET imaging in people with DS. Owing to their extra copy of chromosome 21, which hosts the APP gene, people with DS develop AD pathology at a young age. Researchers recently added tau PET imaging to the Down’s Syndrome Biomarker Initiative (DSBI), a three-year longitudinal study in people with DS (see Dec 2012 news; Rafii et al, 2015). In addition to cognitive tests, as well as amyloid-PET, structural MRI, and FDG-PET imaging acquired previously in the study, the researchers ran tau PET scans on nine participants, averaging 48 years of age, at the two-year mark. These nine displayed no cognitive symptoms of AD yet, though six of them had elevated Aβ at baseline.
At CTAD, Rafii reported that, similar to presymptomatic people in A4, tau accumulation in people with DS was associated with elevated Aβ deposition. The three participants without elevated Aβ did not have tau deposition beyond the medial temporal lobe, whereas those with elevated Aβ displayed the typical “batwing” distribution of neocortical tau (see image below). What’s more, tau accumulation correlated with waning cognitive performance between baseline and two years, as well as with atrophy in the medial temporal lobe.
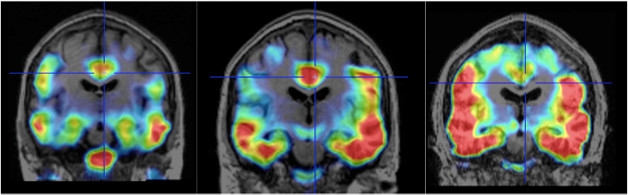
Batwing in Down’s. As tau spreads from the medial temporal lobe into the neocortex, a batwing pattern of tau tracer uptake appears. Scans were conducted on a 48-year-old (left), 50-year-old (middle) and 52-year-old (right) person with DS prior to onset of AD symptoms. [Images courtesy of Michael Rafii, ATRI and UCSD.]
“This fits with the idea that AD biomarkers behave similarly in people with DS as they do in other people with AD,” Rafii told Alzforum. “As such, people with DS are being included in AD prevention trials such as the ACI-24 in DS study," he said.
Lund University’s Schöll compared tau accumulation patterns between early and late-onset AD in the Swedish Biofinder Study. More than 1,600 people participate in this longitudinal study, which aims to identify early biomarkers for a range of neurodegenerative diseases, including Alzheimer’s. Schöll and colleagues conducted tau scans in 17 healthy controls, 16 people with EOAD (defined as onset before age 65), and 17 with LOAD (onset after age 65). Neither AD group harbored familial AD mutations. Unlike Sperling and Rafii’s cohorts, participants in this tau PET sub-study were symptomatic (with average MMSE scores of 21.4 in the EOAD group and 20.6 in the LOAD group) and thus expected to have more advanced tau pathology. Schöll reported that tau distribution was markedly different between the two groups. People with EOAD had tangles in the medial temporal lobe and well outside of it—extending into the posterior, parietal, and occipital cortices. On the other hand, the bulk of tau deposition in people with LOAD remained closer to its origins in the medial temporal lobe, spreading from there into inferior and lateral temporal lobes, but largely avoiding posterior areas.
Schöll speculated that the Aβ-dependent process that drives tau into the neocortex could somehow work differently in EOAD and LOAD even though findings regarding differences in Aβ accumulation between the two groups have been inconsistent. Tau deposition more closely overlapped with atrophy in people with EOAD than LOAD. “The diseases differ both in their relationship between Aβ and tau, and also in the effect of tau on atrophy,” Schöll told Alzforum. Whether these differences relate to the more aggressive nature of early onset disease remains to be seen, he said. Schöll added that clinicians should consider these differences between early and late-onset AD when using tau PET as a selection or monitoring tool in trials, once this pattern has been established further.
How does tau PET compare to measurements of cerebrospinal fluid tau and phospho-tau as a diagnostic or disease progression biomarker? Discussing a poster by Niklas Mattsson, also of Lund, Schöll reported that while both worked as diagnostic markers, tau PET far outperformed CSF total tau or phospho-tau in tracking progression. The researchers conducted tau PET and measured CSF tau/p-tau in 29 people with AD, eight people with MCI, and 17 healthy controls. They found strong correlations between the two modalities in people with low amounts of tau pathology in classic Braak regions, while the association crumbled in people in the later stages of disease, who had a heavy tau burden. One possible interpretation of this finding is that CSF levels of tau and p-tau bump up in the early stages of the disease but then plateau as tau accumulates further, Schöll said, adding that beyond early stages of the disease, tau PET appears to be a far more accurate measure of disease progression.
Filling in for Lund’s Oskar Hansson, who heads the Swedish Biofinder Study but was unable to attend CTAD, Schöll also presented tau imaging data from other neurodegenerative diseases. Originally developed to detect the combined four-repeat and three-repeat tau tangles that occur in AD, the AV-1451 tracer has come up short in other tauopathies that consist of pure 4R or 3R tau. Schöll described recently published data indicating that people with the 4R tauopathy progressive supranuclear palsy (PSP) took up the tracer only in their basal ganglia, an area where background uptake in healthy controls is high. Although tracer uptake in the basal ganglia correlated with PSP severity, Schöll said the high background uptake limited the usefulness of the tracer in people with this disease. There was no uptake in regions of the cortex known to harbor tau aggregates in people with PSP (see Smith et al., 2016).
Results were slightly more encouraging in people with corticobasal degeneration, another 4R tauopathy. Neurodegeneration of the motor cortex and subcortical motor tracts tends to predominate on one side of the brain in CBD, and Schöll reported that tau tracer uptake was indeed highest in those regions on the affected side in six people with the disease. Tracer uptake on the less affected side was much lower, partly overlapping with uptake in healthy controls. Why AV-1451 was taken up in CBD but not PSP is unclear, Hansson told Alzforum by phone later, although he speculated that the tracer might detect pure 4R tau if accumulation surpassed a certain threshold. He stressed that these initial studies are exceedingly small, and much larger series are needed to determine if and how tau imaging is useful in neurodegenerative diseases other than AD. —Jessica Shugart
References
News Citations
- Tau PET Aligns Spread of Pathology with Alzheimer’s Staging
- Brain Imaging Suggests Aβ Unleashes the Deadly Side of Tau
- Solanezumab Selected for Alzheimer’s A4 Prevention Trial
- Natural History-Cum-Trials Initiative, Grants to Boost Down’s Research
Therapeutics Citations
Paper Citations
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239-59. PubMed.
- Rafii MS, Wishnek H, Brewer JB, Donohue MC, Ness S, Mobley WC, Aisen PS, Rissman RA. The down syndrome biomarker initiative (DSBI) pilot: proof of concept for deep phenotyping of Alzheimer's disease biomarkers in down syndrome. Front Behav Neurosci. 2015;9:239. Epub 2015 Sep 14 PubMed.
- Smith R, Schain M, Nilsson C, Strandberg O, Olsson T, Hägerström D, Jögi J, Borroni E, Schöll M, Honer M, Hansson O. Increased basal ganglia binding of (18) F-AV-1451 in patients with progressive supranuclear palsy. Mov Disord. 2016 Oct 6; PubMed.
Further Reading
Papers
- Smith R, Schöll M, Honer M, Nilsson CF, Englund E, Hansson O. Tau neuropathology correlates with FDG-PET, but not AV-1451-PET, in progressive supranuclear palsy. Acta Neuropathol. 2016 Nov 29; PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.