At HAI, Researchers Explore Diagnostic Potential of a Tau Tracer
Quick Links
The accumulation of tau protein in the brain reflects a spectrum of different human neurodegenerative diseases, from those affecting the front of the brain, such as frontotemporal dementia, to those affecting the back, such as posterior cortical atrophy. Scientists are hoping that tau PET imaging will pinpoint those deposits in living people with sufficient accuracy to radically improve the diagnosis, prognosis, and basic understanding of these tauopathies. Alas, this vision may take a bit longer to realize. At this year’s Human Amyloid Imaging conference January 13-15 in Miami Beach, Florida, scientists reported how the leading tau ligand in many cases falls short of distinguishing people with disease from normal healthy controls. HAI made abstracts and keynote talks from Henrik Zetterberg, University of Gothenburg, Sweden, and Charles Duyckaerts, Hôpital Pitié-Salpêtrière, Paris, available here.
Separating Disease from Controls
PET imaging has revolutionized the study of amyloid plaques, and scientists are hoping it will do the same for tau. In the case of Alzheimer’s at least, it may. Neurofibrillary tangles that emerge as AD progresses seem to avidly bind several tau ligands currently in development, and researchers are using tau PET to get a better handle on how Aβ and tau conspire to create havoc in the brain (see Part 1 of this series). As noted time and again at HAI, however, non-AD tauopathies are a different kettle of fish. Researchers noted that while AV1451 seems to bind where one might expect in a given case of tauopathy, it falls short when it comes to being diagnostically useful.

Telltale Tangles.
In one tau mutation carrier, AV1451 binding in the frontal cortex suggests pure frontotemporal dementia (top), while in another PET signals in posterior areas correlate with concomitant Aβ amyloidosis (bottom). [Courtesy of Richard Tsai.]
Richard Tsai, who works with Gil Rabinovici and others at the University of California, San Francisco, examined AV1451 scans from volunteers with different tauopathies and clinical symptoms. His sample included four people with tau mutations, three with a clinical diagnosis of the nonfluent variant of primary progressive aphasia (nfvPPA) and five with corticobasal syndrome (CBS). While the distribution and strength of the tau signal varied, by and large the ligand bound to regions affected by ongoing disease in each case, said Tsai. Interestingly, the imaging brought out subtle differences among the volunteers that fit with their individual pathologies.
For example, two people with different tau mutations bound the ligand in their frontal cortex, consistent with their frontotemporal dementia diagnosis. However, while clinicians gave both of these patients a clinical dementia rating of 2.0, the person carrying a V337M mutation had much higher uptake value ratios (SUVR) for AV1451. This could mean that this mutation leads to the type of paired helical filaments found in AD, said Rabinovici.
Did the PET scan of the other patient underestimate their tau burden? It turned out that this second person carried a P301L mutation, which typically forms straight filaments rather than the paired helical type. This person also had ongoing Aβ amyloidosis as judged by PiB PET. AV1451 bound farther back in the brain, in the parietal and occipital regions, indicating more widespread tau pathology such as might be found in typical Alzheimer’s and suggesting this was a case of mixed dementia.
Two asymptomatic volunteers carrying the same mutation in intron 10 of the tau gene had lower AV1451 uptake; this was consistent with the presence of straight filaments and respective CDR scores of 0.5 and 0.0.
Likewise, among the three nfvPPA patients, PET imaging correlated with subtle differences. Each had a negative PiB PET scan, and their MMSE scores of 26-28 suggested mild cognitive impairment. While all took up a similar amount of AV1451, its distribution varied. In two right-handed volunteers, tracer bound predominantly on the left side of the brain, while the one lefty had a more even distribution between the two hemispheres.
In CBS, AV1451 distribution reflected both right-left asymmetry and underlying Aβ pathology. Of the five patients Tsai scanned, one had a positive PiB PET scan. That person had rampant tau pathology throughout the cortex. Among the other four, AV1451 distributed through the dentate, putamen, pallidum, and white matter. More bound to the left hemisphere in three patients with predominantly right motor symptoms, and to the right hemisphere in a person with left-predominant motor symptoms.
Tsai’s work is good news to the field because it suggests that AV1451 binding reflects the distribution of tau pathology in individual cases of tauopathy. Does that make tau PET a diagnostic tool? It is too early to tell, scientists agreed. In all pure tauopathy cases scanned thus far, much less AV1451 bound in the brain than is typically seen in AD. The consensus at HAI was that the signal may be too weak to distinguish people with those tauopathies from people with age-related tau deposition.
In his presentation, David Jones, working with Bradley Boeve and colleagues at the Mayo Clinic in Rochester, Minnesota, brought home the cold truth by comparing ligand uptake among a larger sample of cognitively normal controls and patients with AD and FTLD. Jones reported that both visual reads and SUVR quantitation of scans did indicate uptake of AV1451 in the brains of five people—three with tau mutations, one with a progranulin mutation, and one with semantic variant PPA. However, comparing their scans with those from 21 AD patients and 120 normal controls revealed that only the semantic variant case bound more ligand than the controls. For the other four patients, SUVRs in both temporal and frontal cortex overlapped with control values (see figure below). Jones therefore concluded that AV1451 binding is insufficient to distinguish FTLD cases.
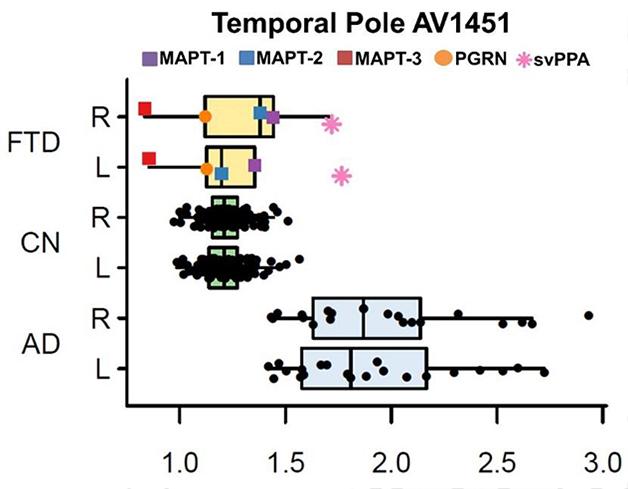
In Alzheimer’s (AD), uptake of the tau tracer AV1451 is higher than in controls. In frontotemporal dementias (FTD) caused by different mutations, it overlaps with controls (CN). [Courtesy of David Jones.]
Off the Mark
Furthermore, Jones’ work emphasized how off-target binding might be a problem. Although FTD patients with progranulin mutations typically have deposits of TDP-43 rather than tau, the PGRN patient Jones scanned bound AV1451 in the frontal lobe. Since previous work suggests that AV1451 has no affinity for TDP-43 deposits (see Feb 2015 conference news), researchers were puzzled by this binding. Rabinovici, Cliff Jack from the Mayo Clinic, Rochester, Minnesota, and Reisa Sperling from Brigham and Women’s Hospital, Boston, all noted that it occurred in an area of ongoing neurodegeneration. They wondered if the ligand might be binding a marker of degeneration other than tau. Jones considered this possible, but would not speculate on what that may be. “We don’t yet understand if this is a non-specific measure of neurodegeneration,” Rabinovici told Alzforum. “There is more work to be done to understand the nature of that signal.”
Diagnosing progressive supranuclear palsy (PSP) based on tau PET may be an even tougher nut to crack. A number of companies are beginning to evaluate tau-based drugs in PSP and would welcome a clear-cut molecular imaging marker for this disease (see Therapeutics database). The problem is that in normal controls, AV1451 binds in some of the very same areas where tau deposits in PSP, including the midbrain and basal ganglia.
Scientists have not given up hope that they may yet tease out a disease-specific signal by homing in on certain regions of the brain. With this in mind, Daniel Schonhaut, who works with Rabinovici at UCSF, compared AV1451 scans of 17 PSP patients at three centers with those from 28 normal controls who tested negative on PiB PET, indicating they had no concomitant amyloid pathology that might complicate the results. Voxel-wise analysis brought out differences between patients and controls. In PSP, AV1451 binding in the pallidum, putamen, dentate nucleus of the cerebellum, and subthalamic nucleus was higher than binding in those regions in controls. In addition, binding in patients trended higher in the substantia nigra, thalamus, caudate, and pons.
The biggest difference occurred in the pallidum, where the SUVR averaged about 2.0, compared to 1.6 in controls. Schonhaut calculated that a threshold for the pallidum of 1.75 could give a sensitivity and specificity of 0.81 and 0.93, respectively. However, there was no correlation between AV1451 uptake and the PSP Rating Scale, which measures disease severity. Longitudinal data are needed to better tease out that relationship, said Schonhaut.
Schonhaut further claimed that AV1451 might prove useful in distinguishing different forms of PSP. Classic PSP, aka Richardson’s syndrome, affects a wider area of the brain, whereas a form called pure akinesia with gait freezing spares the dentate nucleus of the cerebellum. Diagnosing the latter is challenging because symptoms overlap with those of Parkinson’s disease and other dementias, said Rabinovici. Schonhaut reported that among three patients of each type, AV1451 uptake in the dentate nucleus matched their subtype. “While this is a small number of patients, the results are encouraging,” he said.—Tom Fagan
References
News Citations
- Tau Takes Center Stage at 10th Human Amyloid Imaging Conference
- Tau Tracer T807/AV1451 Tracks Neurodegenerative Progression
Mutations Citations
Other Citations
External Citations
Further Reading
No Available Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.