Finally, a Blood Test for Alzheimer’s?
Quick Links
In the quest for the Holy Grail of Alzheimer’s disease biomarkers—a blood test for brain amyloid accumulation—researchers have been stymied. Over and over, studies have failed to find a robust and reproducible relationship between Aβ42 levels in brain and blood, suggesting the latter might not reliably indicate disease. Lo and behold, new research unveiled at the Alzheimer’s Association International Conference, held July 16-20 in London, upends the conventional wisdom. Randall Bateman of Washington University School of Medicine in St. Louis presented a new method for measuring plasma Aβ that relies on mass spectrometry. It greatly increases accuracy and precision over previous antibody-based measures. The method lowers background noise enough to reveal an average 15 percent drop in the plasma ratio of Aβ42/Aβ40 in people with brain amyloid compared to those without, Bateman said. The findings have been replicated in an independent cohort, but the method requires validation in multicenter studies and clinical settings, he added. The data were published in the July 11 Alzheimer’s and Dementia.
The presentation excited researchers in London. “This was by far the biggest news at the meeting. It’s a game-changer with huge implications for prevention trials,” Paul Aisen of the University of Southern California in San Diego told Alzforum. He noted that the high negative rate on amyloid PET scans presents a big problem as prevention studies try to enroll, driving up cost and thereby limiting how large the trial can be. If researchers could screen potential participants with a blood test instead of a scan, costs would drop and trials could screen many more people. Pharma researchers flocked to pick Bateman’s brain after the talk.
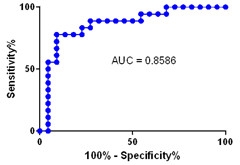
Blood Test for Brain Amyloid.
A representative “area under the curve” graph, showing the specificity and sensitivity of the plasma Aβ42/40 ratio for identifying people with amyloid plaques. [Courtesy of Randall Bateman.]
While previous studies have clashed on whether blood and CSF Aβ correlate (for review see Toledo et al., 2013), Bateman saw encouraging indications that these measures might indeed be linked while studying Aβ production and clearance using stable isotope labeling kinetics (SILK). In this technique, people ingest 13C-leucine, which incorporates into newly made Aβ, allowing researchers to track how quickly the peptide appears and disappears and to follow where it goes (Jun 2006 news). In a separate study, Bateman was surprised to find that between 30 to 50 percent of brain Aβ flowed into the bloodstream (Roberts et al., 2014). Since CSF Aβ levels are 50 times higher than peripheral levels, this suggests the bulk of blood Aβ comes from brain, suggesting to him that blood Aβ levels should reflect those in brain, after all, and that a blood test was worth pursuing.
In his AAIC talk, Bateman described how he applied SILK to study the 24-hour kinetics of Aβ in the blood of 41 cognitively normal older adults, about half of whom had amyloid plaques, as determined by CSF Aβ levels or an amyloid PET scan. In the plaque-positive group, the researchers saw a more rapid appearance and clearance of newly synthesized soluble Aβ42 in plasma that mirrored kinetics in the CSF, strengthening the idea that much of the plasma Aβ comes from brain.
In previous CSF studies, overall clearance of Aβ from the brain has been found to slow in aging and Alzheimer’s disease (Jul 2010 conference news; Dec 2010 news). However, the turnover of the soluble Aβ42 relative to soluble Aβ40 speeds up in people with brain amyloid accumulation, likely reflecting the deposition of Aβ42 into plaques (Patterson et al., 2015; Dec 2016 news). The finding that newly made Aβ42 also appears and disappears faster from plasma in people with plaques confirmed the idea that blood levels indicate amyloid status, and suggested that plaques might lead to low steady-state levels of plasma Aβ42.
Bateman and colleagues then directly measured total Aβ blood levels. They immunoprecipitated the peptides from a 1.7 ml sample of plasma, then digested them with proteases and analyzed the fragments by high-resolution liquid chromatography mass spectrometry. Since their method measures the C-terminal ends of Aβ, it can distinguish between common isoforms such as Aβ42, Aβ40, and Aβ38, but cannot detect N-terminal truncations, Bateman noted. Mass spec analysis does not require isotopic labeling of the peptide.
Just as for Aβ42/Aβ40 in the CSF, this ratio in plasma was lower in the 18 people with brain amyloid than in the 23 without, Bateman reported in London. Plasma and CSF levels correlated with a regression coefficient of 0.70. Samples taken at any time of the day produced similar results. In plasma, an Aβ42/Aβ40 ratio above 0.124 distinguished amyloid-negative from -positive participants with 88 percent accuracy, Bateman found.
To validate these findings, the researchers tested stored plasma samples from a separate cohort of 164 people seen at the Knight Alzheimer’s Disease Research Center at WashU. Again they saw a consistently lower Aβ42/Aβ40 ratio in people with brain amyloid, with a staggering statistical significance of p = 0.00000006.
These samples had been handled extensively, transferred between tubes and frozen. Even so, a cutoff Aβ42/Aβ40 ratio of 0.103 was 76 percent accurate in detecting amyloid accumulation. The researchers determined this cutoff using an external standard curve, so it cannot be directly compared to the value obtained in the smaller study, Bateman noted. In future studies, following standardized protocols could probably improve accuracy, he added. The researchers are also working on lowering the amount of plasma needed for the test.
Eric Reiman of Banner Alzheimer’s Institute, Phoenix, called the work extraordinary. He asked why previous studies failed to find a consistent correlation between Aβ42 levels in CSF and blood. Bateman noted that blood constitutes a much “noisier” environment, chock-full of proteins and ions. Aβ levels in blood are 50-fold lower than in CSF, while other proteins are 1,000-fold higher, making Aβ 50,000 times harder to detect. In addition, because the background solutes are unique for each person, Aβ measurements are affected differently from one individual to the next. For this reason, the coefficient of variation for ELISAs or other antibody-based assays of plasma Aβ averages around 20 percent, which would mask the 15 percent difference in the Aβ42/Aβ40 ratios, Bateman said. By immunoprecipitating Aβ to isolate it from blood, and analyzing it by very-high-resolution mass spec, the background noise falls essentially to zero, he claimed.

Plasma Predicts Brain?
Blood Aβ levels may be specific enough to serve as a potential screening test. In the first study of 41 participants, there was one false negative (triangle above dotted line), but eight false positives (circles below), suggesting such a test could prescreen trial candidates before a PET scan. [Courtesy of Randall Bateman.]
How would such a blood test be used? Bateman noted that the assay produces more false positives than false negatives, with the initial 41-person cohort having eight of the former and only one of the latter. Thus, a negative result could be considered definitive enough to avoid further testing, but a positive result would need to be confirmed by CSF testing or PET scans. Based on the initial data, about two-thirds of people without brain amyloid would test negative on this assay and would not need follow-up PET scans or lumbar punctures.
Bateman suggested that this blood test would serve best as a quick initial screen for people in preclinical or prodromal disease phases. With the costs of mass spec analysis running about one-tenth the price of an amyloid PET scan, that could create savings for large trials, which need to screen thousands of potential participants.
C2N Diagnostics, a company founded by Bateman and others that develops AD assays, is considering how to implement this assay in trials, Bateman noted (Apr 2009 news). Meanwhile, clinical diagnostic use of the test may be possible within a few years, Bateman said.—Madolyn Bowman Rogers
References
News Citations
- CSF Aβ—New Approach Shows Rapid Flux, May Help Evaluate Therapeutics
- Honolulu: Wake-Up Call—Aβ Clearance, Not Production, Awry in AD
- Paper Alert: In Vivo Human Data Shows Reduced Aβ Clearance in AD
- Amyloid Plaques’ Hold on Aβ42 Dampens Peptide’s Daily Rhythm
- Studies Reveal New Hope, Old Problems With AD Biomarkers
Paper Citations
- Toledo JB, Shaw LM, Trojanowski JQ. Plasma amyloid beta measurements - a desired but elusive Alzheimer's disease biomarker. Alzheimers Res Ther. 2013 Mar 8;5(2):8. PubMed.
- Roberts KF, Elbert DL, Kasten TP, Patterson BW, Sigurdson WC, Connors RE, Ovod V, Munsell LY, Mawuenyega KG, Miller-Thomas MM, Moran CJ, Cross DT 3rd, Derdeyn CP, Bateman RJ. Amyloid-β efflux from the central nervous system into the plasma. Ann Neurol. 2014 Dec;76(6):837-44. Epub 2014 Oct 24 PubMed.
- Patterson BW, Elbert DL, Mawuenyega KG, Kasten T, Ovod V, Ma S, Xiong C, Chott R, Yarasheski K, Sigurdson W, Zhang L, Goate A, Benzinger T, Morris JC, Holtzman D, Bateman RJ. Age and amyloid effects on human central nervous system amyloid-beta kinetics. Ann Neurol. 2015 Sep;78(3):439-53. Epub 2015 Jul 20 PubMed.
Further Reading
News
Primary Papers
- Ovod V, Ramsey KN, Mawuenyega KG, Bollinger JG, Hicks T, Schneider T, Sullivan M, Paumier K, Holtzman DM, Morris JC, Benzinger T, Fagan AM, Patterson BW, Bateman RJ. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017 Aug;13(8):841-849. Epub 2017 Jul 19 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.