New Treatment Restores Movement to Paralyzed Rats
Quick Links
Lab rats can overcome hindlimb paralysis induced by spinal injury if they have the right combination of treatments, according to a paper in today’s Science. Researchers at the University of Zürich, Switzerland, combined neurotransmitter injections, electrical stimulation of the spinal cord, and a physical therapy program to restore voluntary movement to the injured animals. The key part of the training was that the rats were motivated to shuffle toward a treat and wore a harness that supported them as they learned to do so. The report shows that, with the right encouragement, the spinal cord can be plastic enough in certain situations to piece together new neural pathways that bypass damaged ones. Elements of this therapy have been tried in people, too, and might help restore movement to those paralyzed by spinal injury or perhaps even disease.
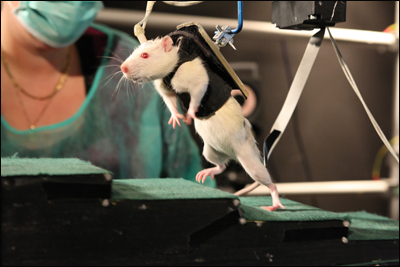
The research group led by Grégoire Courtine (which recently moved to the Swiss Federal Institute of Technology in Lausanne), had restored hindlimb movement to paralyzed rats before, but the ambulation in the earlier work was involuntary. Animals that received injections of serotonin receptor activators and electrical stimulation to the spinal cords trundled along on a treadmill. However, this movement was due entirely to reflex circuits in the spinal cord. The brain was not involved (see ARF related news story on Courtine et al., 2009). In the current work, joint first authors Rubia van den Brand and Janine Heutschi wanted to give the rats control over the movement. They knew a simple treadmill would only prompt the reflex action; to master their own limbs, the rats had to want to walk. That part proved simple: a bit of chocolate or the promise of a cheek rub motivated the rats to stride. To lend a helping hand, the researchers designed a harness, consisting of a vest plus a tail support attached to a robotic arm, to support the rat’s weight when necessary. It works rather like an adult holding a baby’s hands as the child takes its first steps.
First, the researchers caused hindlimb paralysis with one half-section on the left side of thoracic vertebra T7, and another cut on the right side of T10. Since the cord was not completely severed, the surgery created a situation like two parallel highways, each with a roadblock separated by a few miles. To bypass the obstacles, it is not necessary to forge a completely new path. “You simply change to the other track,” said Rómulo Fuentes of the Edmond and Lily Safra International Institute of Neuroscience of Natal, Brazil, who was not involved in the study. As van den Brand and colleagues would discover, neurons indeed were able to create such a bypass under the right conditions.
The first two conditions were previously established. They are an injection of serotonin and dopamine receptor agonists, which primed the neurons to fire, and electrical stimulation of the epidural space around the spinal cord, which start the legs cycling on a treadmill. The third element of the winning mixture—the rats’ own will—required the new robotic support system.
Initially, the researchers used the robotic arm to propel the rats to the treat. This instilled the desire to move forward; the animals waved their front limbs and leaned their heads back in their efforts to walk, van den Brand said. This intention was the crucial element because it engaged the brain. “There would be a moment after three weeks or so when they would be able to produce some locomotor output” on their own, van den Brand said. After a few more weeks, they were able to support their weight, albeit still attached to the harness, scamper across the table, scurry up steps, and hop obstacles of their own volition—all as long as the two other factors, the neurotransmitters and the electrical stimulation, remained.
- Click on the image to launch the video.
Rehab for Rats
Grégoire Courtine of the Swiss Federal Institute of Technology in Lausanne (EPFL) explains how his team restored mobility to paralyzed rats. Image courtesty of EPFL/Grégoire Courtine
Examination of the rats’ spinal cord anatomy confirmed that their neurons had created spinal bypass pathways, with the rehab boosting connections toward and across the lesions. Contrary to many researchers’ expectations about spinal injury repair, the rats did not have to grow novel pathways from the brain to the hindlimbs. Instead, the central nervous system modified existing routes. “The brain figured out a new way,” said Reggie Edgerton of the University of California, Los Angeles, a former mentor of Courtine’s who was not involved in the current work. “The brain can get around these partial lesions if you motivate the animal to try to activate those circuits.”
Whether a similar therapy would work in humans remains to be seen. The treatment depends on having some of the spinal cord stay intact. About a third of people paralyzed by spinal cord damage retain some connections across their lesion, Edgerton said. Case study research from Edgerton’s lab suggests the human brain and spinal cord might be able to bypass an injury. The researchers implanted a stimulating electrode array in the spinal dura of a paraplegic 23-year-old man. After 80 training sessions, he was able to stand for more than four minutes and control limb movements (Harkema et al., 2011).
In this case study, as well as in van den Brand’s rats, turning off the electrical input abolished all the benefit. However, in a second set of experiments, in which the researchers merely bruised the rats’ spinal cords without severing them, the animals were able to regain some hindlimb control even when the stimulator was off, she told ARF. This kind of lesion might be more similar to the majority of human injuries, van den Brand said.
Could rejiggering spinal input become possible for people with stroke or motor neuron disease? Van den Brand did not rule it out, although there are no data to suggest this yet.
The three-part treatment is but one possibility to treat paralysis. Scientists are also working to promote axon regrowth in the hopes of repairing spinal injuries (see ARF related series). On the opposite end of the spectrum, scientists at Brown University in Providence, Rhode Island, aim to bypass damaged nerves altogether by collecting motor cortex signals via a brain-computer interface, then decoding those signals to control a cursor, robot arm, and eventually even the person’s own muscles (see ARF related news story on Hochberg et al., 2012). Courtine’s treatment falls in between, forging new pathways out of existing parts. It is likely that each approach may suit certain types of injury, Edgerton said, and in some cases they may even work best in combination.—Amber Dance
References
News Citations
- Research Brief: Mojo for Motor Neurons
- San Diego: Researchers Rejuvenate Neurons to Bridge Spinal Cord Gaps
- Grasping at the Future of Brain-Computer Interfaces
Paper Citations
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009 Oct;12(10):1333-42. PubMed.
- Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011 Jun 4;377(9781):1938-47. PubMed.
- Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, Haddadin S, Liu J, Cash SS, van der Smagt P, Donoghue JP. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012 May 17;485(7398):372-5. PubMed.
External Citations
Further Reading
Papers
- Xu X, Warrington AE, Bieber AJ, Rodriguez M. Enhancing CNS repair in neurological disease: challenges arising from neurodegeneration and rewiring of the network. CNS Drugs. 2011 Jul;25(7):555-73. PubMed.
- Kadoya K, Tsukada S, Lu P, Coppola G, Geschwind D, Filbin MT, Blesch A, Tuszynski MH. Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron. 2009 Oct 29;64(2):165-72. PubMed.
- Alto LT, Havton LA, Conner JM, Hollis Ii ER, Blesch A, Tuszynski MH. Chemotropic guidance facilitates axonal regeneration and synapse formation after spinal cord injury. Nat Neurosci. 2009 Sep;12(9):1106-13. PubMed.
- Hilliard MA. Axonal degeneration and regeneration: a mechanistic tug-of-war. J Neurochem. 2009 Jan;108(1):23-32. PubMed.
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008 Jan;14(1):69-74. PubMed.
News
- Double Punch Regenerates Injured Spinal Cord, Restores Rat Breathing
- Mend the Gap: Pericytes Form Core of Spinal Cord Scars
- What’s Another Year?—Testing the Limits of Axon Regeneration
- Mind-machine Meld: Brain-computer Interfaces for ALS, Paralysis
- San Diego: Researchers Rejuvenate Neurons to Bridge Spinal Cord Gaps
- Jumpstarting Axon Regeneration?—Not Such a Stretch
- Research Brief: Mojo for Motor Neurons
- Grasping at the Future of Brain-Computer Interfaces
Primary Papers
- van den Brand R, Heutschi J, Barraud Q, DiGiovanna J, Bartholdi K, Huerlimann M, Friedli L, Vollenweider I, Moraud EM, Duis S, Dominici N, Micera S, Musienko P, Courtine G. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012 Jun 1;336(6085):1182-5. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.