It’s a Wrap: Uncovering Signals Behind Myelination
Quick Links
A bare electrical wire is susceptible to corrosion, and so are axons without their layer of myelin insulation, according to some research. Two recent papers help to explain how myelin-producing oligodendrocytes know where and when to wrap axons. According to one study, electrical activity dictates where these cells should myelinate, and the myelination may even be a form of neuronal plasticity that supports learning. The second paper identifies biochemical signals that spark precursors to becoming oligodendrocytes. Together, the findings could help researchers design therapies to jumpstart natural myelination when it is lost due to disease, such as in multiple sclerosis.
Oligodendrocytes do not treat all axons the same; they give some more attention than others. Reporting in the August 4 Sciencexpress, researchers led by Hiroaki Wake, in the laboratory of Doug Fields, posit that firing nerves release signals that promote their own myelination. “This means that active axons would be preferentially myelinated,” said Fields, who heads the team at the National Institute of Child Health and Human Development in Bethesda, Maryland. Further, he suggests that myelination of axons could contribute to neural plasticity.
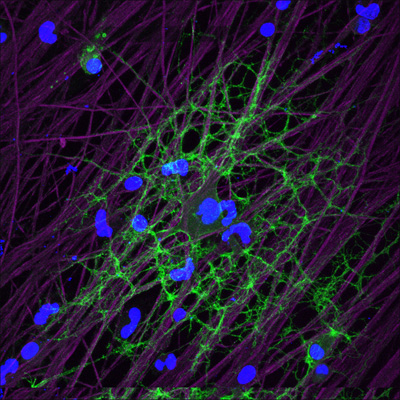
Oligodendrocyte tendrils (green) respond to glutamate released by active axons (purple), ramping up local production of myelin. View larger image. Image credit: R. Douglas Fields and Hiroaki Wake, NIH
But before myelination can happen, oligodendrocyte precursor cells (OPCs) must morph into oligodendrocytes. This process requires dampening of the Wnt pathway—which is involved in embryogenesis and cancer, as well as regulating many normal events in adult animals—according to a paper in the June 26 Nature Neuroscience online. The University of California, San Francisco, study team, led by first author Stephen Fancy and senior author David Rowitch, showed that Wnt pathway member Axin2 regulates this differentiation. They found that a small-molecule inhibitor of Axin2 degradation promoted oligodendrocyte formation and myelination, suggesting a potential route to therapy that might help people with MS by re-insulating their axons.
Myelin Making Memories?
While researchers knew that electrical activity promoted myelination (Demerens et al., 1996), Fields’s work, he said, is the first to show that the stimulation happens locally, within individual oligodendrocyte projections. The researchers also began to untangle the molecular signals responsible. Because oligodendrocytes responded to axon signaling with an uptick in levels of calcium, an important second messenger, Wake and colleagues concluded that the oligodendrocytes were somehow listening in on the nerves’ action potentials.
Initially, the authors wondered whether vesicles releasing neurotransmitters might signal oligodendrocytes to form myelin. They blocked vesicle release, using botulinum toxin, in cultured mouse dorsal root ganglion neurons. Then they washed out excess drug before adding healthy OPCs, allowing them to differentiate into oligodendrocytes, and stimulating the axons. Control neurons received eight times the myelination that toxin-treated ones did, confirming that vesicle release is an essential part of the signal.
To discover the neurotransmitter involved, they treated the cultures with an array of neurotransmitter inhibitors. Blocking either glutamate or adenosine triphosphate (ATP) inhibited the oligodendrocyte calcium wave, indicating that these two molecules signal oligodendrocytes. Since the team was looking for a vesicle-mediated effect, they compared botulinum-treated to untreated control cultures when exposed to ATP or glutamate. ATP’s effects on the oligodendrocytes did not rely on vesicle release; the oligodendrocyte calcium signal continued under botulinum toxin treatment. Glutamate, in contrast, only influenced oligodendrocyte signaling when vesicle trafficking was normal. In addition, glutamate affected calcium signaling only in OPC projections, not in the cell soma. Fields, who imagines oligodendrocytes as many-armed octopi, said, “Glutamate has the earmarks of being a perfect signal to locally control, in each tentacle, where to form myelin.” Thus, they focused on glutamate in further experiments.
Next, the researchers examined the cholesterol-rich lipid rafts that are crucial to the axon-oligodendrocyte junction. Wake labeled a receptor in these rafts, and observed that it moved to the oligodendrocyte surface upon neuron stimulation. They inferred from this that glutamate causes the formation of lipid-containing communication points between axons and oligodendrocytes.
Once they receive the neuron’s encouragement, the oligodendrocyte arms will synthesize copious amounts of myelin. The researchers labeled myelin basic protein and discovered that it was synthesized, locally near the junction, in response to axon stimulation. That area of the cell made myelin even if the researchers blocked all transcription, indicating that the RNA was ready and waiting, in the projections, for the right signals.
The upshot is that the more active the nerve, the more myelination it attracts, making it transmit signals even faster. This positive feedback loop “will have significant effects on information processing,” Fields said. “I think of this as a new form of plasticity and learning” (reviewed in Fields, 2010). Signal-based myelin regulation would allow children's brains to adapt to their environment, he suggested, by strengthening nerves that they use regularly. Even in adults, myelin-rich white matter increases when people learn skills such as reading (Carreiras et al., 2009), juggling (Scholz et al., 2009), or playing a musical instrument (Ullén, 2009). For people who lose myelination—such as those who have suffered injury or have a stroke—physical therapy probably works because the regular exercise of those neurons and axons attracts myelin, making them work better over time, Fields said.
“Previously, there were many gaps in our understanding of exactly how experience might sculpt myelin in the brain,” wrote Heidi Johansen-Berg, a researcher at Oxford University, U.K., and coauthor of the study on juggling, in an e-mail to ARF. “This new study helps to fill in those gaps.” But evidence is still lacking for whether this process happens in vivo, noted Ben Barres of Stanford University, in Palo Alto, California, in an e-mail to ARF. The Fields team is currently setting up animal experiments to test this.
In Alzheimer’s disease, lesions often damage myelin-rich white matter tracts (see ARF related news story on van Norden et al., 2008 and van Straaten et al., 2008), and the current study supports the notion that regular cognitive activity could help people (see ARF related news story on Wilson et al., 2002), perhaps by stimulating continuing myelination of neurons.
A failure of myelination may also contribute to amyotrophic lateral sclerosis. Mouse ALS models carrying human mutant superoxide dismutase-1 (SOD1) exhibit quadruple the number of OPCs in their spinal cords than wild-type animals. These OPCs produce malformed oligodendrocytes that quickly die. Deleting the mutant SOD1 solely in oligodendrocytes extended life. Researchers are not yet sure how oligodendrocytes—and, by extension, myelin—might contribute to the disease (see ARF related news story on Kang et al., 2010).
Researchers studying MS may be particularly interested in these findings. In MS, “there has been a longstanding mystery about why remyelination fails to occur,” Barres wrote. People with MS often can remyelinate nerves in lesions, and they do it quite well, Fancy said (Patani et al., 2007). This accounts for the regular relapses and remissions associated with disease. When the immune system attacks myelin, people with MS might end up in wheelchairs, but as their brain re-insulates those nerves, they can walk again. In people with progressive MS—which often follows relapsing-remitting disease—that remyelination process is ineffective.
“This paper suggests an answer,” Barres wrote. “After demyelination, conduction is blocked, and so axons can no longer conduct electrical signals. If these electrical signals are required for myelination, and by extrapolation, remyelination, then remyelination would not be able to occur.”
The Fancy and Rowitch team offer another explanation for the remyelination block: OPCs in the lesions fail to differentiate into oligodendrocytes. People with MS “actually have a lot of oligodendrocyte progenitors in the lesion,” Fancy said, “but for some reason they seem to be stalled” (Kuhlmann et al., 2008).
Differentiation Delays
In an earlier study, Fancy screened more than 1,000 transcription factors, seeking those that were activated during OPC differentiation and myelination (Fancy et al., 2009). He discovered that Wnt pathway players were activated, and that overactivating the pathway delayed OPC differentiation. In demyelinated lesions, expression of several Wnt pathway members was abnormally high or unusually low, suggesting the pathway was dysregulated.
In the current work, Fancy focused on Wnt pathway member Axin2, which promotes degradation of β-catenin, a key activator of Wnt targets. The team examined the role of Axin2 protein using Axin2 knockout mice. During both developmental myelination and remyelination of lesions in older animals, OPC differentiation was slowed in the knockouts. Thus, the brain was not able to produce oligodendrocytes to myelinate the naked axons, because Axin2 was not present to tone down Wnt pathway activity. “The Axin2 protein is actually needed in the normal process of development and repair,” Fancy theorized. “It is there to damp down the Wnt pathway and allow differentiation to occur.”
If that were so, then increasing Axin2 levels should knock down the Wnt pathway and promote OPC differentiation. Fortunately, another research group had already come up with a molecule that raises Axin2 levels (Huang et al., 2009). The compound XAV939 acts on tankyrases, enzymes that normally promote Axin2 degradation. XAV939 blocks tankyrases, boosting Axin2 protein concentrations—which then minimizes Wnt activity.
Fancy tested the drug in several conditions: cultured OPCs, mouse cerebellar slices, and mice with demyelinated lesions. “In all of these scenarios, the drug improved the extent of oligodendrocyte differentiation,” he reported. The treatment not only boosted oligodendrocyte numbers, it also increased myelin basic protein production and thickened the myelin surrounding axons.
A drug to promote OPC differentiation via the Wnt pathway would be a new kind of MS therapeutic, Fancy said, since current treatments aim to minimize the autoimmune response. Although XAV939 is the first Wnt drug candidate, Fancy noted that, with further study, better clinical candidates might come up. And the implications could be far reaching, noted Patrizia Casaccia, of the Mount Sinai School of Medicine, New York City, in a commentary accompanying the paper in Nature Neuroscience. “The detection of Axin2 mRNA in the brains of adults with multiple sclerosis and in mice with chemically induced demyelination suggests that the Wnt/Axin2/β-catenin signaling pathway may act in any condition associated with myelin damage and subsequent repair,” she wrote.—Amber Dance
References
News Citations
- Sharper Image—Do More Studies Improve Picture of Cognitive Decline?
- Add Mental Exercise to Potential AD Protection
- Fate Accompli—Cells With NG2 Become Oligodendrocytes, Drive ALS?
Paper Citations
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci U S A. 1996 Sep 3;93(18):9887-92. PubMed.
- Fields RD. Neuroscience. Change in the brain's white matter. Science. 2010 Nov 5;330(6005):768-9. PubMed.
- Carreiras M, Seghier ML, Baquero S, Estévez A, Lozano A, Devlin JT, Price CJ. An anatomical signature for literacy. Nature. 2009 Oct 15;461(7266):983-6. PubMed.
- Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009 Nov;12(11):1370-1. PubMed.
- Ullén F. Is activity regulation of late myelination a plastic mechanism in the human nervous system?. Neuron Glia Biol. 2009 May;5(1-2):29-34. PubMed.
- van Norden AG, Fick WF, de Laat KF, van Uden IW, van Oudheusden LJ, Tendolkar I, Zwiers MP, de Leeuw FE. Subjective cognitive failures and hippocampal volume in elderly with white matter lesions. Neurology. 2008 Oct 7;71(15):1152-9. PubMed.
- van Straaten EC, Harvey D, Scheltens P, Barkhof F, Petersen RC, Thal LJ, Jack CR, Decarli C, . Periventricular white matter hyperintensities increase the likelihood of progression from amnestic mild cognitive impairment to dementia. J Neurol. 2008 Sep;255(9):1302-8. PubMed.
- Wilson RS, Mendes de Leon CF, Barnes LL, Schneider JA, Bienias JL, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002 Feb 13;287(6):742-8. PubMed.
- Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010 Nov 18;68(4):668-81. PubMed.
- Patani R, Balaratnam M, Vora A, Reynolds R. Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathol Appl Neurobiol. 2007 Jun;33(3):277-87. PubMed.
- Kuhlmann T, Miron V, Cui Q, Cuo Q, Wegner C, Antel J, Brück W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008 Jul;131(Pt 7):1749-58. PubMed.
- Fancy SP, Baranzini SE, Zhao C, Yuk DI, Irvine KA, Kaing S, Sanai N, Franklin RJ, Rowitch DH. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009 Jul 1;23(13):1571-85. PubMed.
- Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, Hild M, Shi X, Wilson CJ, Mickanin C, Myer V, Fazal A, Tomlinson R, Serluca F, Shao W, Cheng H, Shultz M, Rau C, Schirle M, Schlegl J, Ghidelli S, Fawell S, Lu C, Curtis D, Kirschner MW, Lengauer C, Finan PM, Tallarico JA, Bouwmeester T, Porter JA, Bauer A, Cong F. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature. 2009 Oct 1;461(7264):614-20. PubMed.
Other Citations
Further Reading
Papers
- Desai MK, Guercio BJ, Narrow WC, Bowers WJ. An Alzheimer's disease-relevant presenilin-1 mutation augments amyloid-beta-induced oligodendrocyte dysfunction. Glia. 2011 Apr;59(4):627-40. PubMed.
- Psachoulia K, Jamen F, Young KM, Richardson WD. Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biol. 2009 Nov;5(3-4):57-67. PubMed.
- Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008 Jul;31(7):361-70. PubMed.
- Trajkovic K, Dhaunchak AS, Goncalves JT, Wenzel D, Schneider A, Bunt G, Nave KA, Simons M. Neuron to glia signaling triggers myelin membrane exocytosis from endosomal storage sites. J Cell Biol. 2006 Mar 13;172(6):937-48. PubMed.
- Taveggia C, Thaker P, Petrylak A, Caporaso GL, Toews A, Falls DL, Einheber S, Salzer JL. Type III neuregulin-1 promotes oligodendrocyte myelination. Glia. 2008 Feb;56(3):284-93. PubMed.
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer's disease. Neurobiol Aging. 2004 Jan;25(1):5-18; author reply 49-62. PubMed.
News
- MicroRNAs—Oligarchs of Oligodendrocyte Fate
- Research Brief: Glatiramer Slows Progression From High Risk to MS
- Surprise! Astrocytes Mediate Activity-Stimulated Myelination
- Nogo’s Scope Broadens to Include Multiple Sclerosis
- Add Mental Exercise to Potential AD Protection
- Fate Accompli—Cells With NG2 Become Oligodendrocytes, Drive ALS?
- Sharper Image—Do More Studies Improve Picture of Cognitive Decline?
- Glial γ-Secretase Inhibits Myelination in New CNS Co-culture System
Primary Papers
- Wake H, Lee PR, Fields RD. Control of local protein synthesis and initial events in myelination by action potentials. Science. 2011 Sep 16;333(6049):1647-51. PubMed.
- Fancy SP, Harrington EP, Yuen TJ, Silbereis JC, Zhao C, Baranzini SE, Bruce CC, Otero JJ, Huang EJ, Nusse R, Franklin RJ, Rowitch DH. Axin2 as regulatory and therapeutic target in newborn brain injury and remyelination. Nat Neurosci. 2011 Aug;14(8):1009-16. PubMed.
- Casaccia P. Anti-TANKyrase weapons promote myelination. Nat Neurosci. 2011 Aug;14(8):945-7. PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.